Nanoneedles Induce Targeted siRNA Silencing of p16 in the Human Corneal Endothelium
- PMID: 36253148
- PMCID: PMC9685449
- DOI: 10.1002/advs.202203257
Nanoneedles Induce Targeted siRNA Silencing of p16 in the Human Corneal Endothelium
Abstract
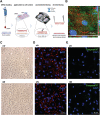
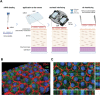
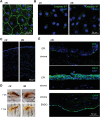
Nanoneedles can target nucleic acid transfection to primary cells at tissue interfaces with high efficiency and minimal perturbation. The corneal endothelium is an ideal target for nanoneedle-mediated RNA interference therapy aimed at enhancing its proliferative capacity, necessary for tissue regeneration. This work develops a strategy for siRNA nanoninjection to the human corneal endothelium. Nanoneedles can deliver p16-targeting siRNA to primary human corneal endothelial cells in vitro without toxicity. The nanoinjection of siRNA induces p16 silencing and increases cell proliferation, as monitored by ki67 expression. Furthermore, siRNA nanoinjection targeting the human corneal endothelium is nontoxic ex vivo, and silences p16 in transfected cells. These data indicate that nanoinjection can support targeted RNA interference therapy for the treatment of endothelial corneal dysfunction.
Keywords: gene therapy; nanoneedles; porous silicon; regenerative medicine; siRNA.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Joyce N. C., Navon S. E., Roy S., Zieske J. D., Invest. Ophthalmol. Visual Sci. 1996, 37, 1566. - PubMed
-
- Gain P., Jullienne R., He Z., Aldossary M., Acquart S., Cognasse F., Thuret G., JAMA Ophthalmol. 2016, 134, 167. - PubMed
-
- Van Den Bogerd B., Dhubhghaill S. N., Koppen C., Tassignon M.‐J. C., Zakaria N., Surv. Ophthalmol. 2018, 63, 149. - PubMed
-
- Català P., Thuret G., Skottman H., Mehta J. S., Parekh M., Dhubhghailll S. N., Collin R. W. J., Nuijts R. M. M. A., Ferrari S., LaPointe V. L. S., Dickman M. M., Prog. Retinal Eye Res. 2021, 87, 100987. - PubMed
-
- Kampik D., Ali R. R., Larkin D. F., Exp. Eye Res. 2012, 95, 54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
