Lecanemab, Aducanumab, and Gantenerumab - Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer's Disease
- PMID: 36253511
- PMCID: PMC10119362
- DOI: 10.1007/s13311-022-01308-6
Lecanemab, Aducanumab, and Gantenerumab - Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer's Disease
Abstract
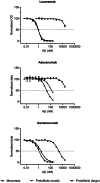
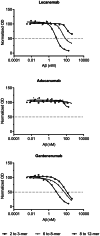
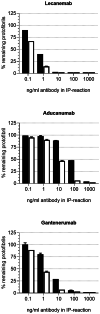
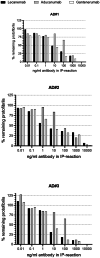
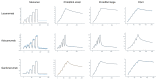
Immunotherapy against amyloid-beta (Aβ) is a promising option for the treatment of Alzheimer's disease (AD). Aβ exists as various species, including monomers, oligomers, protofibrils, and insoluble fibrils in plaques. Oligomers and protofibrils have been shown to be toxic, and removal of these aggregates might represent an effective treatment for AD. We have characterized the binding properties of lecanemab, aducanumab, and gantenerumab to different Aβ species with inhibition ELISA, immunodepletion, and surface plasmon resonance. All three antibodies bound monomers with low affinity. However, lecanemab and aducanumab had very weak binding to monomers, and gantenerumab somewhat stronger binding. Lecanemab was distinctive as it had tenfold stronger binding to protofibrils compared to fibrils. Aducanumab and gantenerumab preferred binding to fibrils over protofibrils. Our results show different binding profiles of lecanemab, aducanumab, and gantenerumab that may explain clinical results observed for these antibodies regarding both efficacy and side effects.
Keywords: Aducanumab; Amyloid-beta species; Gantenerumab; Lecanemab; Therapeutic antibodies.
© 2022. The Author(s).
Conflict of interest statement
LS, MJ, PN, HL, FE, GO, CM, and LL are employees and shareholders of BioArctic. LL is a co‐founder and board member of BioArctic.
Figures

References
-
- Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem. 1997;272(35):22364–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

