Allergen immunotherapy: past, present and future
- PMID: 36253555
- PMCID: PMC9575636
- DOI: 10.1038/s41577-022-00786-1
Allergen immunotherapy: past, present and future
Abstract
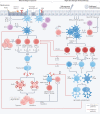
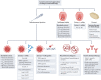
Allergen immunotherapy is a form of therapeutic vaccination for established IgE-mediated hypersensitivity to common allergen sources such as pollens, house dust mites and the venom of stinging insects. The classical protocol, introduced in 1911, involves repeated subcutaneous injection of increasing amounts of allergen extract, followed by maintenance injections over a period of 3 years, achieving a form of allergen-specific tolerance that provides clinical benefit for years after its discontinuation. More recently, administration through the sublingual route has emerged as an effective, safe alternative. Oral immunotherapy for peanut allergy induces effective 'desensitization' but not long-term tolerance. Research and clinical trials over the past few decades have elucidated the mechanisms underlying immunotherapy-induced tolerance, involving a reduction of allergen-specific T helper 2 (TH2) cells, an induction of regulatory T and B cells, and production of IgG and IgA 'blocking' antibodies. To better harness these mechanisms, novel strategies are being explored to achieve safer, effective, more convenient regimens and more durable long-term tolerance; these include alternative routes for current immunotherapy approaches, novel adjuvants, use of recombinant allergens (including hypoallergenic variants) and combination of allergens with immune modifiers or monoclonal antibodies targeting the TH2 cell pathway.
© 2022. Springer Nature Limited.
Conflict of interest statement
S.R.D. reports research grants from the Immune Tolerance Network, National Institutes of Allergy and Infectious Diseases USA, Medical Research Council UK and GlaxoSmithKline; has received lecture fees from Abbott laboratories, ALK, Allergopharma, Pneumo Update GmbH and Stallergenes Greer; and has received consultancy fees from ALK, ANGANY Inc. and Revolo Biotherapeutics. M.H.S. reports grants from Leti, Regeneron, Merck, ANGANY Inc., Allergy Therapeutics and the Immune Tolerance Network; reports personal fees from Allergopharma; and reports grants and personal fees from ALK and Allergy Therapeutics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

