Human cerebral organoids - a new tool for clinical neurology research
- PMID: 36253568
- PMCID: PMC9576133
- DOI: 10.1038/s41582-022-00723-9
Human cerebral organoids - a new tool for clinical neurology research
Abstract
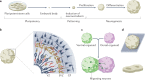
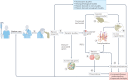
The current understanding of neurological diseases is derived mostly from direct analysis of patients and from animal models of disease. However, most patient studies do not capture the earliest stages of disease development and offer limited opportunities for experimental intervention, so rarely yield complete mechanistic insights. The use of animal models relies on evolutionary conservation of pathways involved in disease and is limited by an inability to recreate human-specific processes. In vitro models that are derived from human pluripotent stem cells cultured in 3D have emerged as a new model system that could bridge the gap between patient studies and animal models. In this Review, we summarize how such organoid models can complement classical approaches to accelerate neurological research. We describe our current understanding of neurodevelopment and how this process differs between humans and other animals, making human-derived models of disease essential. We discuss different methodologies for producing organoids and how organoids can be and have been used to model neurological disorders, including microcephaly, Zika virus infection, Alzheimer disease and other neurodegenerative disorders, and neurodevelopmental diseases, such as Timothy syndrome, Angelman syndrome and tuberous sclerosis. We also discuss the current limitations of organoid models and outline how organoids can be used to revolutionize research into the human brain and neurological diseases.
© 2022. Springer Nature Limited.
Conflict of interest statement
J.A.K. is on the supervisory and scientific advisory board of a:head bio AG (aheadbio.com) and is an inventor on several patents relating to cerebral organoids. O.L.E. declares no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

