Direct delivery and fast-treated Agrobacterium co-culture (Fast-TrACC) plant transformation methods for Nicotiana benthamiana
- PMID: 36253612
- PMCID: PMC9839554
- DOI: 10.1038/s41596-022-00749-9
Direct delivery and fast-treated Agrobacterium co-culture (Fast-TrACC) plant transformation methods for Nicotiana benthamiana
Abstract
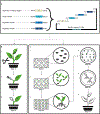
There is an expanding need to modify plant genomes to create new plant germplasm that advances both basic and applied plant research. Most current methods for plant genome modification involve regenerating plants from genetically modified cells in tissue culture, which is technically challenging, expensive and time consuming, and works with limited plant species or genotypes. Herein, we describe two Agrobacterium-based methods for creating genetic modifications on either sterilely grown or soil-grown Nicotiana benthamiana plants. These methods use developmental regulators (DRs), gene products that influence cell division and differentiation, to induce de novo meristems. Genome editing reagents, such as the RNA-guided endonuclease Cas9, may be co-delivered with the DRs to create shoots that transmit edits to the next generation. One method, called fast-treated Agrobacterium co-culture (Fast-TrACC), delivers DRs to seedlings grown aseptically; meristems that produce shoots and ultimately whole plants are induced. The other approach, called direct delivery (DD), involves delivering DRs to soil-grown plants from which existing meristems have been removed; the DRs promote the formation of new shoots at the wound site. With either approach, if transgene cassettes and/or gene editing reagents are provided, these induced, de novo meristems may be transgenic, edited or both. These two methods offer alternative approaches for generating novel plant germplasm that are cheaper and less technically challenging and take less time than standard approaches. The whole procedure from transfer DNA (T-DNA) assembly to recovery of edited plants can be completed in ~70 d for both DD and Fast-TrACC.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
M.F.M., R.A.N. and D.F.V. are named inventors on a patent application describing the DD and Fast-TrACC methods, which was filed by the University of Minnesota. D.F.V. consults for Calyxt, an agricultural biotechnology company that uses gene editing to create new crop varieties. All other authors have no competing interests.
Figures




References
-
- Nelson-Vasilchik K, Hague J, Mookkan M, Zhang ZJ & Kausch A Transformation of recalcitrant sorghum varieties facilitated by Baby Boom and Wuschel2. Curr. Protoc. Plant Biol. 3, e20076 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

