Estimation of skeletal kinematics in freely moving rodents
- PMID: 36253644
- PMCID: PMC9636019
- DOI: 10.1038/s41592-022-01634-9
Estimation of skeletal kinematics in freely moving rodents
Abstract
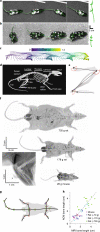
Forming a complete picture of the relationship between neural activity and skeletal kinematics requires quantification of skeletal joint biomechanics during free behavior; however, without detailed knowledge of the underlying skeletal motion, inferring limb kinematics using surface-tracking approaches is difficult, especially for animals where the relationship between the surface and underlying skeleton changes during motion. Here we developed a videography-based method enabling detailed three-dimensional kinematic quantification of an anatomically defined skeleton in untethered freely behaving rats and mice. This skeleton-based model was constrained using anatomical principles and joint motion limits and provided skeletal pose estimates for a range of body sizes, even when limbs were occluded. Model-inferred limb positions and joint kinematics during gait and gap-crossing behaviors were verified by direct measurement of either limb placement or limb kinematics using inertial measurement units. Together we show that complex decision-making behaviors can be accurately reconstructed at the level of skeletal kinematics using our anatomically constrained model.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

