Adverse events of iron and/or erythropoiesis-stimulating agent therapy in preoperatively anemic elective surgery patients: a systematic review
- PMID: 36253838
- PMCID: PMC9578279
- DOI: 10.1186/s13643-022-02081-5
Adverse events of iron and/or erythropoiesis-stimulating agent therapy in preoperatively anemic elective surgery patients: a systematic review
Abstract
Background: Iron supplementation and erythropoiesis-stimulating agent (ESA) administration represent the hallmark therapies in preoperative anemia treatment, as reflected in a set of evidence-based treatment recommendations made during the 2018 International Consensus Conference on Patient Blood Management. However, little is known about the safety of these therapies. This systematic review investigated the occurrence of adverse events (AEs) during or after treatment with iron and/or ESAs.
Methods: Five databases (The Cochrane Library, MEDLINE, Embase, Transfusion Evidence Library, Web of Science) and two trial registries (ClinicalTrials.gov, WHO ICTRP) were searched until 23 May 2022. Randomized controlled trials (RCTs), cohort, and case-control studies investigating any AE during or after iron and/or ESA administration in adult elective surgery patients with preoperative anemia were eligible for inclusion and judged using the Cochrane Risk of Bias tools. The GRADE approach was used to assess the overall certainty of evidence.
Results: Data from 26 RCTs and 16 cohort studies involving a total of 6062 patients were extracted, on 6 treatment comparisons: (1) intravenous (IV) versus oral iron, (2) IV iron versus usual care/no iron, (3) IV ferric carboxymaltose versus IV iron sucrose, (4) ESA+iron versus control (placebo and/or iron, no treatment), (5) ESA+IV iron versus ESA+oral iron, and (6) ESA+IV iron versus ESA+IV iron (different ESA dosing regimens). Most AE data concerned mortality/survival (n=24 studies), thromboembolic (n=22), infectious (n=20), cardiovascular (n=19) and gastrointestinal (n=14) AEs. Very low certainty evidence was assigned to all but one outcome category. This uncertainty results from both the low quantity and quality of AE data due to the high risk of bias caused by limitations in the study design, data collection, and reporting.
Conclusions: It remains unclear if ESA and/or iron therapy is associated with AEs in preoperatively anemic elective surgery patients. Future trial investigators should pay more attention to the systematic collection, measurement, documentation, and reporting of AE data.
Keywords: Adverse events; Anemia; Blood transfusion; Elective surgery; Erythropoiesis-stimulating agents; Hematinics; Iron; Preoperative care; Systematic review.
© 2022. The Author(s).
Conflict of interest statement
Financial competing interests directly related to this review: PMM received personal fees from Ethos Srl (Advisory Board on PBM) and SUMMEET Srl (Speaker in a meeting on PBM). PM received grants from Vifor Pharma, SerumWerke Bernburg, csl Behring, Fresenius Medical, and B.Baun. PM received personal fees from Vifor Pharma and Pharmacosmos. HV, JL, BA, EDB, GB, JG, VC, YO, and PV declared not having any relevant direct financial competing interest.
Financial competing interests not directly related to this review: HV, JL, BA, EDB, VC, and PV are employees of Belgian Red Cross-Flanders, which is responsible for supplying adequate quantities of safe blood products to hospitals in Flanders and Brussels on a continuous basis and is being paid for this activity by the Ministry of Social Affairs. Belgian Red Cross-Flanders received a grant from the European Blood Alliance to conduct this review. PMM received personal fees from Editree Srl (creation of training course on porphyria), Eleuthera Srl (AHP Advisory Board), Collage SpA (speaker in a meeting on Erythrocytosis), IQVIA (Advisory Board on porphyria), and Alnylam (Advisory Board on porphyria). GB, JG, PM, and YO declared not having any other financial competing interest.
Non-financial competing interests: JL, HVR, BA, GB, PMM, YO, EDB, VC, and PV declared not having any non-financial competing interests. PM declared to be involved in the implementation of PBM programs. JG declared to be involved in The Danish Health Authority-National Clinical Guideline-Indication for Transfusion with Blood Components-Copenhagen 2018 (
Figures
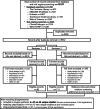
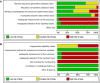

 Low risk of bias.
Low risk of bias.
 Unclear risk of bias.
Unclear risk of bias.
 High risk of bias
High risk of bias

References
-
- (WHO) WHO . WHO global forum for blood safety: patient blood management. 2011.
-
- Van Remoortel H, Aranko K, Mueller MM, De Buck E, Devine D, Follea G, et al. The systematic use of evidence-based methodologies and technologies enhances shared decision-making in the 2018 International Consensus Conference on Patient Blood Management. Vox Sang. 2020;115(1):60–71. doi: 10.1111/vox.12852. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

