Serum IGF-1 Scores and Clinical Outcomes in the Phase III IMbrave150 Study of Atezolizumab Plus Bevacizumab versus Sorafenib in Patients with Unresectable Hepatocellular Carcinoma
- PMID: 36254201
- PMCID: PMC9569161
- DOI: 10.2147/JHC.S369951
Serum IGF-1 Scores and Clinical Outcomes in the Phase III IMbrave150 Study of Atezolizumab Plus Bevacizumab versus Sorafenib in Patients with Unresectable Hepatocellular Carcinoma
Abstract
Purpose: Child-Turcotte-Pugh class A (CTP-A) in unresectable hepatocellular carcinoma (HCC) is the standard criterion for active therapy and clinical trial enrollment. We hypothesized that insulin-like growth factor-1 (IGF-1) derived scores may provide improved survival prediction over CTP classification. This study aimed to evaluate the potential prognostic and predictive effects of IGF-1 derived scores in the phase III IMbrave150 study.
Patients and methods: Baseline and on-treatment serum IGF-1 levels from 371 patients were subjected to central analysis. Patients' IGF-1 score (1/2/3) and IGF-CTP score (A/B/C) were determined based on pre-specified cutoffs. Outcomes were analyzed by baseline and by on-treatment changes of the IGF-1 and IGF-CTP scores within and between the two treatment arms. The interaction between these scores and outcomes was assessed using univariate and multivariate analyses.
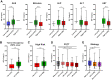
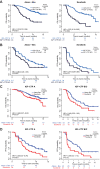
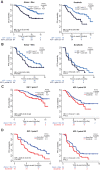
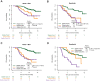
Results: Baseline IGF-CTP score (A vs B/C) showed prognostic significance for OS in both the atezolizumab-bevacizumab (hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.20-0.56; P<0.001) and sorafenib (HR, 0.32; 95% CI, 0.16-0.65; P=0.002) arms. Baseline IGF-1 score (1 vs 2/3) also showed prognostic significance for OS in both the atezolizumab-bevacizumab (HR, 0.33; 95% CI, 0.20-0.55; P<0.001) and sorafenib (HR, 0.48; 95% CI, 0.26-0.89; P=0.02) arms. HRs for PFS were consistent with those for OS. No significant predictive effects were observed for either score between the two arms. Kinetic analysis revealed that patients with increased IGF-1 score (1-> 2/3) at 3 weeks post treatment had shorter OS than patients with stable IGF-1 score of 1 in both the atezolizumab-bevacizumab (HR, 3.70; 95% CI, 1.56-8.77; P=0.003) and sorafenib (HR, 5.83; 95% CI, 1.88-18.12; P=0.0023) arms.
Conclusion: Baseline and kinetic change of IGF-CTP and IGF-1 scores are independent prognostic factors for patients with unresectable HCC treated with atezolizumab-bevacizumab or sorafenib. These novel scores may provide improved patient stratification in future HCC clinical trials. IMbrave150 ClincialTrials.gov number, NCT03434379.
Keywords: HCC; IGF-CTP score; immunotherapy; prognostic biomarker.
© 2022 Kaseb et al.
Conflict of interest statement
A.O.K. has received honoraria from Bayer Health, Bristol Myers Squibb, Eisai, Exelixis, Genentech/Roche, and Merck; has received consulting fees from Bayer Health, Bristol Myers Squibb, Eisai, Exelixis, Genentech/Roche, and Merck; has received institutional research funding from Adaptimmune, Bayer/Onyx, Bristol Myers Squibb, Genentech, Hengrui Pharmaceutical, and Merck; and has received travel, accommodations, and other expense support from Bayer/Onyx, Bristol Myers Squibb, Exelixis, and Merck. Y.G. is an employee of Genentech and holds stock or other ownership interests in F. Hoffmann-La Roche. B.G.Y. has no conflicts of interest to disclose. A.R.A. and S.L. are employees of Genentech and hold stock or other ownership interests in F. Hoffmann-La Roche. E.H. has no conflicts of interest to disclose. H.C.T. has received honoraria from Roche, MSD Merck, Ipsen, and AstraZeneca. W.V. and Y.W. are employees of Genentech and hold stock or other ownership interests in F. Hoffmann-La Roche. The authors report no other conflicts of interest in this work.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

