Engineered skeletal muscle recapitulates human muscle development, regeneration and dystrophy
- PMID: 36254806
- PMCID: PMC9745484
- DOI: 10.1002/jcsm.13094
Engineered skeletal muscle recapitulates human muscle development, regeneration and dystrophy
Abstract
Background: Human pluripotent stem cell-derived muscle models show great potential for translational research. Here, we describe developmentally inspired methods for the derivation of skeletal muscle cells and their utility in skeletal muscle tissue engineering with the aim to model skeletal muscle regeneration and dystrophy in vitro.
Methods: Key steps include the directed differentiation of human pluripotent stem cells to embryonic muscle progenitors followed by primary and secondary foetal myogenesis into three-dimensional muscle. To simulate Duchenne muscular dystrophy (DMD), a patient-specific induced pluripotent stem cell line was compared to a CRISPR/Cas9-edited isogenic control line.
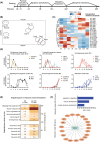
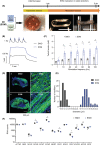
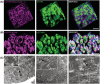
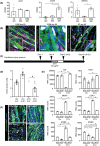
Results: The established skeletal muscle differentiation protocol robustly and faithfully recapitulates critical steps of embryonic myogenesis in two-dimensional and three-dimensional cultures, resulting in functional human skeletal muscle organoids (SMOs) and engineered skeletal muscles (ESMs) with a regeneration-competent satellite-like cell pool. Tissue-engineered muscle exhibits organotypic maturation and function (up to 5.7 ± 0.5 mN tetanic twitch tension at 100 Hz in ESM). Contractile performance could be further enhanced by timed thyroid hormone treatment, increasing the speed of contraction (time to peak contraction) as well as relaxation (time to 50% relaxation) of single twitches from 107 ± 2 to 75 ± 4 ms (P < 0.05) and from 146 ± 6 to 100 ± 6 ms (P < 0.05), respectively. Satellite-like cells could be documented as largely quiescent PAX7+ cells (75 ± 6% Ki67- ) located adjacent to muscle fibres confined under a laminin-containing basal membrane. Activation of the engineered satellite-like cell niche was documented in a cardiotoxin injury model with marked recovery of contractility to 57 ± 8% of the pre-injury force 21 days post-injury (P < 0.05 compared to Day 2 post-injury), which was completely blocked by preceding irradiation. Absence of dystrophin in DMD ESM caused a marked reduction of contractile force (-35 ± 7%, P < 0.05) and impaired expression of fast myosin isoforms resulting in prolonged contraction (175 ± 14 ms, P < 0.05 vs. gene-edited control) and relaxation (238 ± 22 ms, P < 0.05 vs. gene-edited control) times. Restoration of dystrophin levels by gene editing rescued the DMD phenotype in ESM.
Conclusions: We introduce human muscle models with canonical properties of bona fide skeletal muscle in vivo to study muscle development, maturation, disease and repair.
Keywords: Duchenne muscular dystrophy; hypaxial dermomyotome; limb muscle; satellite cells; skeletal muscle organoid; somite; tissue engineering.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The University of Göttingen has filed a patent on skeletal muscle generation listing M. Shahriyari, W‐H.Z. and M.T. as inventors (WO 2021/074126A1). W‐H.Z. is founder, shareholder and advisor of myriamed GmbH, MyriaMeat GmbH and Repairon GmbH. M.T. is founder of MyriaMeat GmbH and advisor of myriamed GmbH and Repairon GmbH.
Figures



References
-
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 1987;51:987–1000. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

