Ocular manifestations of central insulin resistance
- PMID: 36255004
- PMCID: PMC9827783
- DOI: 10.4103/1673-5374.355765
Ocular manifestations of central insulin resistance
Abstract
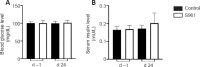
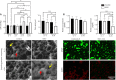
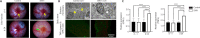
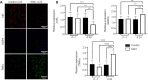
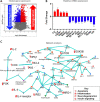
Central insulin resistance, the diminished cellular sensitivity to insulin in the brain, has been implicated in diabetes mellitus, Alzheimer's disease and other neurological disorders. However, whether and how central insulin resistance plays a role in the eye remains unclear. Here, we performed intracerebroventricular injection of S961, a potent and specific blocker of insulin receptor in adult Wistar rats to test if central insulin resistance leads to pathological changes in ocular structures. 80 mg of S961 was stereotaxically injected into the lateral ventricle of the experimental group twice at 7 days apart, whereas buffer solution was injected to the sham control group. Blood samples, intraocular pressure, trabecular meshwork morphology, ciliary body markers, retinal and optic nerve integrity, and whole genome expression patterns were then evaluated. While neither blood glucose nor serum insulin level was significantly altered in the experimental or control group, we found that injection of S961 but not buffer solution significantly increased intraocular pressure at 14 and 24 days after first injection, along with reduced porosity and aquaporin 4 expression in the trabecular meshwork, and increased tumor necrosis factor α and aquaporin 4 expression in the ciliary body. In the retina, cell density and insulin receptor expression decreased in the retinal ganglion cell layer upon S961 injection. Fundus photography revealed peripapillary atrophy with vascular dysregulation in the experimental group. These retinal changes were accompanied by upregulation of pro-inflammatory and pro-apoptotic genes, downregulation of anti-inflammatory, anti-apoptotic, and neurotrophic genes, as well as dysregulation of genes involved in insulin signaling. Optic nerve histology indicated microglial activation and changes in the expression of glial fibrillary acidic protein, tumor necrosis factor α, and aquaporin 4. Molecular pathway architecture of the retina revealed the three most significant pathways involved being inflammation/cell stress, insulin signaling, and extracellular matrix regulation relevant to neurodegeneration. There was also a multimodal crosstalk between insulin signaling derangement and inflammation-related genes. Taken together, our results indicate that blocking insulin receptor signaling in the central nervous system can lead to trabecular meshwork and ciliary body dysfunction, intraocular pressure elevation, as well as inflammation, glial activation, and apoptosis in the retina and optic nerve. Given that central insulin resistance may lead to neurodegenerative phenotype in the visual system, targeting insulin signaling may hold promise for vision disorders involving the retina and optic nerve.
Keywords: brain; ciliary bodies; gene expression; inflammation; insulin receptor; insulin resistance; intraocular pressure; neurodegeneration; optic nerve; retina; retinal ganglion cells; trabecular meshwork.
Conflict of interest statement
None
Figures

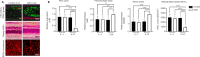

References
-
- Barishak YR. Embryology of the eye and its adnexae. Dev Ophthalmol. 1992;24:1–142. - PubMed
LinkOut - more resources
Full Text Sources

