Casirivimab and Imdevimab Treatment Reduces Viral Load and Improves Clinical Outcomes in Seropositive Hospitalized COVID-19 Patients with Nonneutralizing or Borderline Neutralizing Antibodies
- PMID: 36255239
- PMCID: PMC9765482
- DOI: 10.1128/mbio.01699-22
Casirivimab and Imdevimab Treatment Reduces Viral Load and Improves Clinical Outcomes in Seropositive Hospitalized COVID-19 Patients with Nonneutralizing or Borderline Neutralizing Antibodies
Abstract
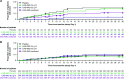
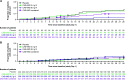
We conducted a post hoc analysis in seropositive patients who were negative or borderline for functional neutralizing antibodies (NAbs) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at baseline from a phase 1, 2, and 3 trial of casirivimab and imdevimab (CAS+IMD) treatment in hospitalized coronavirus disease 2019 (COVID-19) patients on low-flow or no supplemental oxygen prior to the emergence of Omicron-lineage variants. Patients were randomized to a single dose of 2.4 g CAS+IMD, 8.0 g CAS+IMD, or placebo. Patients seropositive for anti-SARS-CoV-2 antibodies at baseline were analyzed by their baseline neutralizing antibody status. At baseline, 20.6% (178/864) of seropositive patients were negative or borderline for neutralizing antibodies, indicating negative or very low functionally neutralizing anti-SARS-CoV-2 antibodies. CAS+IMD reduced viral load in patients who were negative or borderline for neutralizing antibodies versus placebo, but not in patients who were positive for neutralizing antibodies. In patients who were negative or borderline for neutralizing antibodies, we observed a trend in reduction of the proportion of patients who died or required mechanical ventilation, as well as in all-cause mortality, by day 29 with CAS+IMD versus placebo. The proportions of patients who died or required mechanical ventilation from days 1 to 29 were 19.1% in the placebo group and 10.9% in the CAS+IMD combined-dose group, and the proportions of patients who died (all-cause mortality) from days 1 to 29 were 16.2% in the placebo group and 9.1% in the CAS+IMD combined-dose group. In patients who were positive for neutralizing antibodies, no measurable harm or benefit was observed in either the proportion of patients who died or required mechanical ventilation or the proportion of patients who died (all-cause mortality). In hospitalized COVID-19 patients on low-flow or no supplemental oxygen, CAS+IMD reduced viral load, the risk of death or mechanical ventilation, and all-cause mortality in seropositive patients who were negative or borderline for neutralizing antibodies. IMPORTANCE The clinical benefit of CAS+IMD in hospitalized seronegative patients with COVID-19 has previously been demonstrated, although these studies observed no clinical benefit in seropositive patients. As the prevalence of SARS-CoV-2-seropositive individuals rises due to both vaccination and previous infection, it is important to understand whether there is a subset of hospitalized patients with COVID-19 with antibodies against SARS-CoV-2 who could benefit from anti-SARS-CoV-2 monoclonal antibody treatment. This post hoc analysis demonstrates that there is a subset of hospitalized seropositive patients with inadequate SARS-CoV-2-neutralizing antibodies (i.e., those who were negative or borderline for neutralizing antibodies) who may still benefit from CAS+IMD treatment if infected with a susceptible SARS-CoV-2 variant. Therefore, utilizing serostatus alone to guide treatment decisions for patients with COVID-19 may fail to identify those seropositive patients who could benefit from anti-SARS-CoV-2 monoclonal antibody therapies known to be effective against circulating strains, dependent upon how effectively their endogenous antibodies neutralize SARS-CoV-2.
Trial registration: ClinicalTrials.gov NCT04426695.
Keywords: COVID-19; anti-SARS-CoV-2 serostatus; casirivimab and imdevimab; hospitalized; monoclonal antibodies; neutralizing antibodies.
Conflict of interest statement
The authors declare a conflict of interest. A.T.H. is a Regeneron Pharmaceuticals, Inc. employee/stockholder; a former Pfizer employee and current stockholder; has a pending patent application with Regeneron Pharmaceuticals, Inc.; and reports grants from BARDA. S.S.-K., S.E.M., S.A., J.M., R.B., A.M., P.D., and D.M.W. are Regeneron Pharmaceuticals, Inc. employees/stockholders; and report grants from BARDA. E.M. reports payments to his institution received from NIH/NIAID, NIH/NIGMS, SciClone Pharmaceuticals, Regeneron Pharmaceuticals, Inc., Pfizer, Chemic Labs/KODA Therapeutics, Cidara, and Leidos Biomedical Research Inc./NCI; and reports grants from BARDA. G.D.Y. is a Regeneron Pharmaceuticals, Inc. employee/stockholder; has issued (U.S. Patent Nos. 10,787,501, 10,954,289, and 10,975,139) and pending patent applications with Regeneron Pharmaceuticals, Inc.; and reports grants from BARDA. G.A.H. and J.D.H. are Regeneron Pharmaceuticals, Inc. employees/stockholders; and have a pending patent application, with Regeneron Pharmaceuticals, Inc.; and report grants from BARDA.
Figures


References
-
- Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, Gallagher E, Thelwall S, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown K, Hopkins S, Chand M, Ladhani SN, Ramsay M, Lopez Bernal J. 2022. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med 386:340–350. doi:10.1056/NEJMoa2115481. - DOI - PMC - PubMed
-
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown KE, Hopkins S, Chand M, Ramsay M. 2021. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 385:585–594. doi:10.1056/NEJMoa2108891. - DOI - PMC - PubMed
-
- Thomas SJ, Moreira ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU, C4591001 Clinical Trial Group . 2021. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 385:1761–1773. doi:10.1056/NEJMoa2110345. - DOI - PMC - PubMed
-
- Ward H, Cooke G, Whitaker M, Redd R, Eales O, Brown JC, Collet K, Cooper E, Daunt A, Jones K, Moshe M, Willicombe M, Day S, Atchison C, Darzi A, Donnelly CA, Riley S, Ashby D, Barclay WS, Elliott P. 2021. REACT-2 round 5: increasing prevalence of SARS-CoV-2 antibodies demonstrate impact of the second wave and of vaccine roll-out in England. medRxiv doi:10.1101/2021.02.26.21252512. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

