Cryo-EM Structure of Gokushovirus ΦEC6098 Reveals a Novel Capsid Architecture for a Single-Scaffolding Protein, Microvirus Assembly System
- PMID: 36255280
- PMCID: PMC9645218
- DOI: 10.1128/jvi.00990-22
Cryo-EM Structure of Gokushovirus ΦEC6098 Reveals a Novel Capsid Architecture for a Single-Scaffolding Protein, Microvirus Assembly System
Abstract
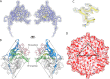
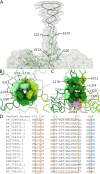
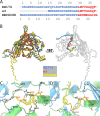
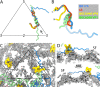
Ubiquitous and abundant in ecosystems and microbiomes, gokushoviruses constitute a Microviridae subfamily, distantly related to bacteriophages ΦX174, α3, and G4. A high-resolution cryo-EM structure of gokushovirus ΦEC6098 was determined, and the atomic model was built de novo. Although gokushoviruses lack external scaffolding and spike proteins, which extensively interact with the ΦX174 capsid protein, the core of the ΦEC6098 coat protein (VP1) displayed a similar structure. There are, however, key differences. At each ΦEC6098 icosahedral 3-fold axis, a long insertion loop formed mushroom-like protrusions, which have been noted in lower-resolution gokushovirus structures. Hydrophobic interfaces at the bottom of these protrusions may confer stability to the capsid shell. In ΦX174, the N-terminus of the capsid protein resides directly atop the 3-fold axes of symmetry; however, the ΦEC6098 N-terminus stretched across the inner surface of the capsid shell, reaching nearly to the 5-fold axis of the neighboring pentamer. Thus, this extended N-terminus interconnected pentamers on the inside of the capsid shell, presumably promoting capsid assembly, a function performed by the ΦX174 external scaffolding protein. There were also key differences between the ΦX174-like DNA-binding J proteins and its ΦEC6098 homologue VP8. As seen with the J proteins, C-terminal VP8 residues were bound into a pocket within the major capsid protein; however, its N-terminal residues were disordered, likely due to flexibility. We show that the combined location and interaction of VP8's C-terminus and a portion of VP1's N-terminus are reminiscent of those seen with the ΦX174 and α3 J proteins. IMPORTANCE There is a dramatic structural and morphogenetic divide within the Microviridae. The well-studied ΦX174-like viruses have prominent spikes at their icosahedral vertices, which are absent in gokushoviruses. Instead, gokushovirus major coat proteins form extensive mushroom-like protrusions at the 3-fold axes of symmetry. In addition, gokushoviruses lack an external scaffolding protein, the more critical of the two ΦX174 assembly proteins, but retain an internal scaffolding protein. The ΦEC6098 virion suggests that key external scaffolding functions are likely performed by coat protein domains unique to gokushoviruses. Thus, within one family, different assembly paths have been taken, demonstrating how a two-scaffolding protein system can evolve into a one-scaffolding protein system, or vice versa.
Keywords: DNA-binding protein; Microviridae; cryo-EM; gokushovirus; microvirus; mushroom spike.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Structural studies of bacteriophage alpha3 assembly.J Mol Biol. 2003 Jan 3;325(1):11-24. doi: 10.1016/s0022-2836(02)01201-9. J Mol Biol. 2003. PMID: 12473449 Free PMC article.
-
Atomic structure of the degraded procapsid particle of the bacteriophage G4: induced structural changes in the presence of calcium ions and functional implications.J Mol Biol. 1996 Mar 8;256(4):736-50. doi: 10.1006/jmbi.1996.0121. J Mol Biol. 1996. PMID: 8642594
-
The microviridae: Diversity, assembly, and experimental evolution.Virology. 2016 Apr;491:45-55. doi: 10.1016/j.virol.2016.01.020. Epub 2016 Feb 11. Virology. 2016. PMID: 26874016 Review.
-
Identification of an interacting coat-external scaffolding protein domain required for both the initiation of phiX174 procapsid morphogenesis and the completion of DNA packaging.J Virol. 2005 Jun;79(11):6751-6. doi: 10.1128/JVI.79.11.6751-6756.2005. J Virol. 2005. PMID: 15890913 Free PMC article.
-
Breaking Symmetry in Viral Icosahedral Capsids as Seen through the Lenses of X-ray Crystallography and Cryo-Electron Microscopy.Viruses. 2018 Feb 7;10(2):67. doi: 10.3390/v10020067. Viruses. 2018. PMID: 29414851 Free PMC article. Review.
Cited by
-
Integrating Sequence- and Structure-Based Similarity Metrics for the Demarcation of Multiple Viral Taxonomic Levels.Viruses. 2025 Apr 29;17(5):642. doi: 10.3390/v17050642. Viruses. 2025. PMID: 40431654 Free PMC article.
-
The Structure of Spiroplasma Virus 4: Exploring the Capsid Diversity of the Microviridae.Viruses. 2024 Jul 9;16(7):1103. doi: 10.3390/v16071103. Viruses. 2024. PMID: 39066266 Free PMC article.
-
A stargate mechanism of Microviridae genome delivery unveiled by cryogenic electron tomography.bioRxiv [Preprint]. 2024 Jun 11:2024.06.11.598214. doi: 10.1101/2024.06.11.598214. bioRxiv. 2024. Update in: mBio. 2025 Apr 9;16(4):e0371324. doi: 10.1128/mbio.03713-24. PMID: 38915634 Free PMC article. Updated. Preprint.
-
Penton blooming, a conserved mechanism of genome delivery used by disparate microviruses.mBio. 2025 Apr 9;16(4):e0371324. doi: 10.1128/mbio.03713-24. Epub 2025 Mar 19. mBio. 2025. PMID: 40105351 Free PMC article.
-
Structural basis for Salmonella infection by two Microviridae phages.Commun Biol. 2025 Aug 6;8(1):1166. doi: 10.1038/s42003-025-08595-7. Commun Biol. 2025. PMID: 40770068 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

