Electrofreezing of Liquid Ammonia
- PMID: 36255376
- PMCID: PMC9619927
- DOI: 10.1021/acs.jpclett.2c02576
Electrofreezing of Liquid Ammonia
Abstract
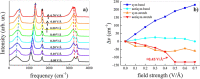
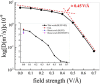
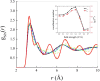
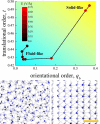
Here we prove that, in addition to temperature and pressure, another important thermodynamic variable permits the exploration of the phase diagram of ammonia: the electric field. By means of (path integral) ab initio molecular dynamics simulations, we predict that, upon applying intense electric fields on ammonia, the electrofreezing phenomenon occurs, leading the liquid toward a novel ferroelectric solid phase. This study proves that electric fields can generally be exploited as the access key to otherwise-unreachable regions in phase diagrams, unveiling the existence of new condensed-phase structures. Furthermore, the reported findings have manifold practical implications, from the safe storage and transportation of ammonia to the understanding of the solid structures this compound forms in planetary contexts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Yang B.; Ding W.; Zhang H.; Zhang S. Recent progress in electrochemical synthesis of ammonia from nitrogen: strategies to improve the catalytic activity and selectivity. Energy Environ. Sci. 2021, 14, 672–687. 10.1039/D0EE02263B. - DOI
-
- Hofstadter M. D.; Muhleman D. O. Latitudinal variations of ammonia in the atmosphere of Uranus: An analysis of microwave observations. Icarus 1989, 81, 396–412. 10.1016/0019-1035(89)90060-2. - DOI
-
- Lindal G. F. The atomosphere of Neptune: an analysis of radio occultation data acquired with Voyager 2. Astron. J. 1992, 103, 967–982. 10.1086/116119. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

