NET-Triggered NLRP3 Activation and IL18 Release Drive Oxaliplatin-Induced Peripheral Neuropathy
- PMID: 36255412
- PMCID: PMC9716254
- DOI: 10.1158/2326-6066.CIR-22-0197
NET-Triggered NLRP3 Activation and IL18 Release Drive Oxaliplatin-Induced Peripheral Neuropathy
Abstract
Oxaliplatin is an antineoplastic agent frequently used in the treatment of gastrointestinal tumors. However, it causes dose-limiting sensorimotor neuropathy, referred to as oxaliplatin-induced peripheral neuropathy (OIPN), for which there is no effective treatment. Here, we report that the elevation of neutrophil extracellular traps (NET) is a pathologic change common to both cancer patients treated with oxaliplatin and a murine model of OIPN. Mechanistically, we found that NETs trigger NLR family pyrin domain containing 3 (NLRP3) inflammasome activation and the subsequent release of IL18 by macrophages, resulting in mechanical hyperalgesia. In NLRP3-deficient mice, the mechanical hyperalgesia characteristic of OIPN in our model was reduced. In addition, in the murine model, treatment with the IL18 decoy receptor IL18BP prevented the development of OIPN. We further showed that eicosapentaenoic acid (EPA) reduced NET formation by suppressing the LPS-TLR4-JNK pathway and thereby abolished NLRP3 inflammasome activation and the subsequent secretion of IL18, which markedly prevented oxaliplatin-induced mechanical hyperalgesia in mice. These results identify a role for NET-triggered NLRP3 activation and IL18 release in the development of OIPN and suggest that utilizing IL18BP and EPA could be effective treatments for OIPN.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures
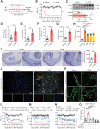
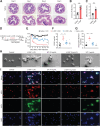
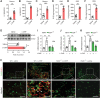

![Figure 5. NLRP3 activation and subsequent IL18 release contribute to oxaliplatin-induced mechanical hyperalgesia. A, The level of ROS in the DRG at day 14 after the initiation of L-OHP treatment was assessed by DCFH-DA staining (n = 8). B and C, The level of ROS in BMDMs stimulated with L-OHP (1, 5, or 10 μmol/L) for 1 hour was assessed by calculating the ratio of DCFH-DA–positive cells to 10,000 cells through flow cytometry. BMDMs stimulated with rotenone (10 μmol/L, 6 hours) were used as a positive control (n = 4). D–K, BMDMs were stimulated with NETs for 3 hours and then stimulated with L-OHP (10 μmol/L) or left unstimulated for 1 hour. LPS (1 μg/mL)-primed BMDMs were treated with ATP (1.5 mmol/L) as a positive control (n = 3). Western blot was used to assess the activation of NLRP3 inflammasome–related proteins [NLRP3, Pro-IL1β, Pro-caspase-1, Caspase-1 (p20), IL1β] in BMDMs treated with NETs and/or L-OHP in both culture supernatants [caspase-1 (p20), IL1β] and cell lysates (NLRP3, Pro-IL1β, Pro-caspase-1; D-I). IL1β or IL18 in supernatants were analyzed by ELISA (J and K). L–Q, WT and Pad4−/− mice were treated with L-OHP (3 mg/kg) for 5 consecutive days to induce mechanical hyperalgesia. The protein levels of NLRP3, caspase-1 (p20), IL1β, IL18, and H3Cit in the DRG were evaluated on the 14th day by western blot (n = 3). R, The levels of IL18 in WT and Pad4−/− mice plasma were evaluated by ELISA at day 14 after the initiation of L-OHP treatment (n = 8). S, WT and Nlrp3−/− mice were treated with L-OHP (3 mg/kg) for 5 consecutive days for a total dose of 15 mg/kg to induce mechanical hyperalgesia. The mechanical pain threshold was tested for 14 days by the von Frey test (n = 8). T, NLRP3 inhibitor MCC950 (20 mg/kg, i.p.) was administered one day before L-OHP administration until the end of 14 days (n = 8). The mechanical pain threshold was tested for 14 days by the von Frey test. U, Mice were treated with IL18BP (200 μg/kg, i.p.) or anakinra (2.5 mg/kg, i.p.) 1 day before L-OHP administration. The mechanical pain threshold was tested for 14 days by the von Frey test (n = 8). V, IL18 (20 ng per mouse, i.p.) was administered one day before L-OHP administration to Nlrp3−/− mice until the end of 7 days. The mechanical pain threshold was measured for 7 days (n = 6). W, The phosphorylation level of Glu2B in the spinal cord was evaluated on the 14th day after the initiation of L-OHP treatment by western blot (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data are shown as mean ± SEM.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/82ed/9716254/a7406810826e/1542fig5.gif)
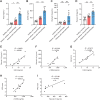
![Figure 7. EPA prevents OIPN by suppressing the formation of NETs and abolishing the activation of NLRP3 inflammasome. A, NET formation was quantitatively detected by Sytox Green staining. Bone marrow–derived neutrophils were incubated with LPS (1 μg/mL) for 4 hours. DHA (20 μmol/L) or EPA (20 μmol/L) was added to the medium 1 hour before LPS. Then, the cells were stained with 1 μmol/L Sytox Green for 15 minutes, and fluorescence intensity was captured at an emission peak of 523 nm when excited by a 488 nm argon-ion laser (n = 8). B, Immunofluorescence staining of NETs was performed: H3Cit (green), MPO (red) and DAPI (blue). The images are representative of three independent experiments. Scale bar, 50 μm. C, Bone marrow–derived neutrophils were incubated with LPS (10 μg/mL) for 1 hour, and EPA (20 μmol/L) was added to the medium for 1 hour before LPS treatment. The phosphorylation of JNK was analyzed by western blotting (n = 3). D–G, BMDMs transfected with Arrb2-targeting siRNA were pretreated with EPA (20 μmol/L) for 1 hour before NET stimulation. BMDMs were stimulated with NETs for 3 hours in presence of EPA, and then oxaliplatin (10 μmol/L) was added for 1 hour. Western blot was used to test the activation of NLRP3 inflammasomes–related proteins [Pro-IL18, caspase-1 (p20)] in BMDMs in both culture supernatants (Pro-IL18) and cell lysates [caspase-1 (p20); n = 3)]. D–F, IL18 in supernatants was analyzed by ELISA (n = 3). G. H–O, EPA (1 g/kg, i.g.) was administered by gavage 5 days before the initiation of L-OHP treatment to the 14th day. The levels of cfDNA and H3Cit in the plasma were evaluated by the Quant-iTPico green dsDNA kit and the H3Cit ELISA kit (n = 8; H–I). The mechanical pain threshold was tested for 14 days by the von Frey test (n = 8; J). The protein levels of H3Cit, NLRP3, caspase-1(p20) and IL18 in the DRG were evaluated on the 14th day after the initiation of L-OHP treatment by western blot (n = 3; K–O). P, Schematic illustration indicating that oxaliplatin-induced NETs contribute to the development of OIPN via NLRP3 activation and IL18 release. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data, mean ± SEM.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/82ed/9716254/ddf5ad6af790/1542fig7.gif)
References
-
- Sereno M, Gutiérrez-Gutiérrez G, Gómez-Raposo C, López-Gómez M, Merino-Salvador M, Tébar FZ, et al. Oxaliplatin induced-neuropathy in digestive tumors. Crit Rev Oncol Hematol 2014;89:166–78. - PubMed
-
- Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother 2005;39:128–35. - PubMed
-
- Kang L, Tian Y, Xu S, Chen H. Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment. J. Neurol 2021;268:3269–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

