Sex-specific newborn screening for X-linked adrenoleukodystrophy
- PMID: 36256460
- PMCID: PMC10092852
- DOI: 10.1002/jimd.12571
Sex-specific newborn screening for X-linked adrenoleukodystrophy
Abstract
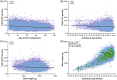
Males with X-linked adrenoleukodystrophy (ALD) are at high risk for developing adrenal insufficiency and/or progressive leukodystrophy (cerebral ALD) at an early age. Pathogenic variants in ABCD1 result in elevated levels of very long-chain fatty acids (VLCFA), including C26:0-lysophosphatidylcholine (C26:0-LPC). Newborn screening for ALD enables prospective monitoring and timely therapeutic intervention, thereby preventing irreversible damage and saving lives. The Dutch Health Council recommended to screen only male newborns for ALD without identifying untreatable conditions associated with elevated C26:0-LPC, like Zellweger spectrum disorders and single peroxisomal enzyme defects. Here, we present the results of the SCAN (Screening for ALD in the Netherlands) study which is the first sex-specific newborn screening program worldwide. Males with ALD are identified based on elevated C26:0-LPC levels, the presence of one X-chromosome and a variant in ABCD1, in heel prick dried bloodspots. Screening of 71 208 newborns resulted in the identification of four boys with ALD who, following referral to the pediatric neurologist and confirmation of the diagnosis, enrolled in a long-term follow-up program. The results of this pilot show the feasibility of employing a boys-only screening algorithm that identifies males with ALD without identifying untreatable conditions. This approach will be of interest to countries that are considering ALD newborn screening but are reluctant to identify girls with ALD because for girls there is no direct health benefit. We also analyzed whether gestational age, sex, birth weight and age at heel prick blood sampling affect C26:0-LPC concentrations and demonstrate that these covariates have a minimal effect.
Keywords: ABCD1; C26:0-LPC; X-chromosome; adrenoleukodystrophy; dried bloodspots; heel prick; newborn screening; sex-specific.
© 2022 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Monique Albersen, Samantha van der Beek, Inge Dijkstra, Mariëlle Alders, Rinse Barendsen, Jet Bliek, Anita Boelen, Merel Ebberink, Sacha Ferdinandusse, Susan Goorden, Annemieke Heijboer, Mandy Jansen, Yorrick Jaspers, Ingrid Metgod, Gajja Salomons, Rendelien Verschoof‐Puite, Wouter Visser, and Eugènie Dekkers declare that they have no conflict of interest. Frédéric Vaz has received consulting fees from Scenic Biotech outside the submitted work. Marc Engelen has received unrestricted research grants from Minoryx, SwanBio Therapeutics, Bluebird Bio, and AutoBahn Therapeutics separate from the submitted work; has received consulting fees from Minoryx, Swanbio Therapeutics, Bluebird Bio, AutoBahn Therapeutics, and Poxel for scientific advising outside the submitted work; participates in advisory board the United Leukodystrophy Foundation (unpaid). Stephan Kemp has received unrestricted research grant support from Bluebird Bio and Swanbio Therapeutics separate from the submitted work; has received consulting fees from Poxel and Swanbio Therapeutics for scientific advising outside the submitted work; participates in advisory boards for ALD Connect (unpaid), the European Leukodystrophy Association (unpaid), Alex, The Leukodystrophy Charity (unpaid), and the United Leukodystrophy Foundation (unpaid).
Figures
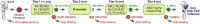

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

