Hevin/Sparcl1 drives pathological pain through spinal cord astrocyte and NMDA receptor signaling
- PMID: 36256481
- PMCID: PMC9746899
- DOI: 10.1172/jci.insight.161028
Hevin/Sparcl1 drives pathological pain through spinal cord astrocyte and NMDA receptor signaling
Abstract
High endothelial venule protein/SPARC-like 1 (hevin/Sparcl1) is an astrocyte-secreted protein that regulates synapse formation in the brain. Here we show that astrocytic hevin signaling plays a critical role in maintaining chronic pain. Compared with WT mice, hevin-null mice exhibited normal mechanical and heat sensitivity but reduced inflammatory pain. Interestingly, hevin-null mice have faster recovery than WT mice from neuropathic pain after nerve injury. Intrathecal injection of WT hevin was sufficient to induce persistent mechanical allodynia in naive mice. In hevin-null mice with nerve injury, adeno-associated-virus-mediated (AAV-mediated) re-expression of hevin in glial fibrillary acidic protein-expressing (GFAP-expressing) spinal cord astrocytes could reinstate neuropathic pain. Mechanistically, hevin is crucial for spinal cord NMDA receptor (NMDAR) signaling. Hevin-potentiated N-Methyl-D-aspartic acid (NMDA) currents are mediated by GluN2B-containing NMDARs. Furthermore, intrathecal injection of a neutralizing Ab against hevin alleviated acute and persistent inflammatory pain, postoperative pain, and neuropathic pain. Secreted hevin that was detected in mouse cerebrospinal fluid (CSF) and nerve injury significantly increased CSF hevin abundance. Finally, neurosurgery caused rapid and substantial increases in SPARCL1/HEVIN levels in human CSF. Collectively, our findings support a critical role of hevin and astrocytes in the maintenance of chronic pain. Neutralizing of secreted hevin with monoclonal Ab may provide a new therapeutic strategy for treating acute and chronic pain and NMDAR-medicated neurodegeneration.
Keywords: Neurological disorders; Neuroscience.
Conflict of interest statement
Figures


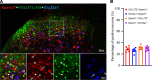
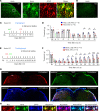

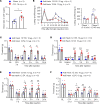

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

