Cobalt-Catalyzed Deaminative Amino- and Alkoxycarbonylation of Aryl Trialkylammonium Salts Promoted by Visible Light
- PMID: 36256542
- PMCID: PMC9729412
- DOI: 10.1002/anie.202210772
Cobalt-Catalyzed Deaminative Amino- and Alkoxycarbonylation of Aryl Trialkylammonium Salts Promoted by Visible Light
Abstract
Catalytic carbonylations of aryl electrophiles via C(sp2 )-N cleavage remains a significant challenge. Herein, we demonstrate an aminocarbonylation of aniline-derived trialkylammonium salts promoted by visible light with a simple cobalt catalyst. The reaction proceeds under mild conditions suitable for late-stage functionalization and is amenable to telescoped carbonylations directly from anilines. A range of alkylamines are successful partners, and alkoxycarbonylation is also demonstrated. Mechanistic studies and DFT calculations support a novel mechanism for catalytic carbonylations of aryl electrophiles involving a key visible light-induced carbonyl photodissociation.
Keywords: Amines; Carbonylation; Cobalt; Synthetic Methods; Visible Light.
© 2022 Wiley-VCH GmbH.
Figures

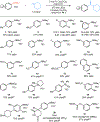
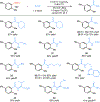




References
-
- Ricci A, Amino Group Chemistry: From Synthesis to the Life Sciences, Wiley, 2008;
- Horton DA, Bourne GT, Smythe ML, Chem. Rev 2003, 103, 893–930; - PubMed
- Czarnik AW, Acc. Chem. Res 1996, 29, 112–113; - PubMed
- Quintás-Cardama A, Kantarjian H, Cortes J, Nat. Rev. Drug Discov 2007, 6, 834–848; - PubMed
- Singer RA, Sadighi JP, Buchwald SL, J. Am. Chem. Soc 1998, 120, 213–214.
-
- Wang Z-X, Yang B, Org. Biomol. Chem 2020, 18, 1057–1072; - PubMed
- Wenkert E, Han A-L, Jenny C-J, J. Chem. Soc., Chem. Commun 1988, 975–976;
- Blakey SB, MacMillan DWC, J. Am. Chem. Soc 2003, 125, 6046–6047; - PubMed
- Chen Q, Gao F, Tang H, Yao M, Zhao Q, Shi Y, Dang Y, Cao C, ACS Catal. 2019, 9, 3730–3736;
- Yang Z-K, Wang D-Y, Minami H, Ogawa H, Ozaki T, Saito T, Miyamoto K, Wang C, Uchiyama M, Chem. Eur. J 2016, 22, 15693–15699; - PubMed
- Xie L-G, Wang Z-X, Angew. Chem. Int. Ed 2011, 50, 4901–4904; Angew. Chem., 2011, 123, 5003–5006; - PubMed
- Zhang H, Hagihara S, Itami K, Chem. Eur. J 2015, 21, 16796–16800; - PubMed
- Zhang X-Q, Wang Z-X, Org. Biomol. Chem 2014, 12, 1448–1453; - PubMed
- Chen H, Yang H, Li N, Xue X, He Z, Zeng Q, Org. Process Res. Dev 2019, 23, 1679–1685;
- Yi Y-Q-Q, Yang W-C, Zhai D-D, Zhang X-Y, Li S-Q, Guan B-T, Chem. Commun 2016, 52, 10894–10897; - PubMed
- Rand AW, Montgomery J, Chem. Sci 2019, 10, 5338–5344; - PMC - PubMed
- Zhu F, Tao J-L, Wang Z-X, Org. Lett 2015, 17, 4926–4929; - PubMed
- Li J, Wang Z-X, Org. Biomol. Chem 2016, 14, 7579–7584. - PubMed
-
- Kong X, Chen Y, Chen X, Lu Z-X, Wang W, Ni S-F, Cao Z-Y, Org. Lett 2022, 24, 2137–2142. - PubMed
-
- Roglans A, Pla-Quintana A, Moreno-Mañas M, Chem. Rev 2006, 106, 4622–4643; - PubMed
- Gosset C, Pellegrini S, Jooris R, Bousquet T, Pelinski L, Adv. Synth. Catal 2018, 360, 3401–3405;
- Majek M, Jacobi von Wangelin A, Angew. Chem. Int. Ed 2015, 54, 2270–2274; Angew. Chem. 2015, 127, 2298 –2302; - PubMed
- Xu J-X, Franke R, Wu X-F, Org. Biomol. Chem 2018, 16, 6180–6182. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

