Dose-sparing effect of two adjuvant formulations with a pandemic influenza A/H7N9 vaccine: A randomized, double-blind, placebo-controlled, phase 1 clinical trial
- PMID: 36256646
- PMCID: PMC9578608
- DOI: 10.1371/journal.pone.0274943
Dose-sparing effect of two adjuvant formulations with a pandemic influenza A/H7N9 vaccine: A randomized, double-blind, placebo-controlled, phase 1 clinical trial
Abstract
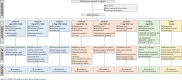
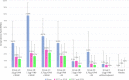
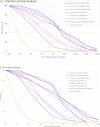
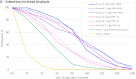
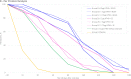
The emergence of potentially pandemic viruses has resulted in preparedness efforts to develop candidate vaccines and adjuvant formulations. We evaluated the dose-sparing effect and safety of two distinct squalene-based oil-in-water adjuvant emulsion formulations (IB160 and SE) with influenza A/H7N9 antigen. This phase I, randomized, double-blind, placebo-controlled, dose-finding trial (NCT03330899), enrolled 432 healthy volunteers aged 18 to 59. Participants were randomly allocated to 8 groups: 1A) IB160 + 15μg H7N9, 1B) IB160 + 7.5μg H7N9, 1C) IB160 + 3.75μg H7N9, 2A) SE + 15μg H7N9, 2B) SE + 7.5μg H7N9, 2C) SE + 3.75μg H7N9, 3) unadjuvanted vaccine 15μg H7N9 and 4) placebo. Immunogenicity was evaluated through haemagglutination inhibition (HI) and microneutralization (MN) tests. Safety was evaluated by monitoring local and systemic, solicited and unsolicited adverse events (AE) and reactions (AR) 7 and 28 days after each study injection, respectively, whereas serious adverse events (SAE) were monitored up to 194 days post-second dose. A greater increase in antibody geometric mean titers (GMT) was observed in groups receiving adjuvanted vaccines. Vaccinees receiving IB160-adjuvanted formulations showed the greatest response in group 1B, which induced an HI GMT increase of 4.7 times, HI titers ≥40 in 45.2% of participants (MN titers ≥40 in 80.8%). Vaccinees receiving SE-adjuvanted vaccines showed the greatest response in group 2A, with an HI GMT increase of 2.5 times, HI titers ≥40 in 22.9% of participants (MN titers ≥40 in 65.7%). Frequencies of AE and AR were similar among groups. Pain at the administration site and headache were the most frequent local and systemic solicited ARs. The vaccine candidates were safe and the adjuvanted formulations have a potential dose-sparing effect on immunogenicity against influenza A/H7N9. The magnitude of this effect could be further explored.
Conflict of interest statement
Daniela H Silveira, Juliana Y K Viscondi, Patrícia Emilia Braga, Maria da Graça Salomão, Vera Lúcia Gattás, Maria Beatriz B Lucchesi, Mayra M M de Oliveira, Marcelo E Koike, Milena A Akamatsu and Paulo Lee Ho are employees of Instituto Butantan-Fundação Butantan. Marilda A M Siqueira, Cristiana C Garcia, and Milene D Miranda are employees of Fiocruz. Maria do Carmo S T Timenetsky and Terezinha M Paiva are employees of Instituto Adolfo Lutz. Alexander R Precioso, Tazio Vanni, Beatriz C Thomé, Roberta O Piorelli, Anderson da Silva, Eduardo A Adami, Joane P Santos, and Gabriella Mondini are former employees of Instituto Butantan-Fundação Butantan. Esper G Kallas, Lucia M A Campos, and Eduardo B Coelho received research-grant from Fundação Butantan for their roles as principal investigators. Erin Sparrow, Martin Friede, Christopher B Fox. Anna Marie Beckmann, and Chuong Huynh are scientific and financial sponsors. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Mulligan MJ, Bernstein DI, Winokur P, Rupp R, Anderson E, Rouphael N, et al.. DMID 13–0032 H7N9 Vaccine Study Group. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA. 2014. Oct 8;312(14):1409–19. doi: 10.1001/jama.2014.12854 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

