Evaluation of the systemic and mucosal immune response induced by COVID-19 and the BNT162b2 mRNA vaccine for SARS-CoV-2
- PMID: 36256664
- PMCID: PMC9578597
- DOI: 10.1371/journal.pone.0263861
Evaluation of the systemic and mucosal immune response induced by COVID-19 and the BNT162b2 mRNA vaccine for SARS-CoV-2
Abstract
Background: The currently used SARS-CoV-2 mRNA vaccines have proven to induce a strong and protective immune response. However, functional relevance of vaccine-generated antibodies and their temporal progression are still poorly understood. Thus, the central aim of this study is to gain a better understanding of systemic and mucosal humoral immune response after mRNA vaccination with BNT162b2.
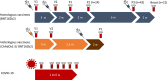
Methods: We compared antibody production against the S1 subunit and the RBD of the SARS-CoV-2 spike protein in sera of BNT162b2 vaccinees, heterologous ChAdOx1-S/BNT162b2 vaccinees and COVID-19 patients. We monitored the neutralizing humoral response against SARS-CoV-2 wildtype strain and three VOCs over a period of up to eight months after second and after a subsequent third vaccination.
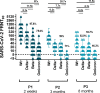
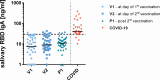
Results: In comparison to COVID-19 patients, vaccinees showed higher or similar amounts of S1- and RBD-binding antibodies but similar or lower virus neutralizing titers. Antibodies peaked two weeks after the second dose, followed by a decrease three and eight months later. Neutralizing antibodies (nAbs) poorly correlated with S1-IgG levels but strongly with RBD-IgGAM titers. After second vaccination we observed a reduced vaccine-induced neutralizing capacity against VOCs, especially against the Omicron variant. Compared to the nAb levels after the second vaccination, the neutralizing capacity against wildtype strain and VOCs was significantly enhanced after third vaccination. In saliva samples, relevant levels of RBD antibodies were detected in convalescent samples but not in vaccinees.
Conclusions: Our data demonstrate that BNT162b2 vaccinated individuals generate relevant nAbs titers, which begin to decrease within three months after immunization and show lower neutralizing potential against VOCs as compared to the wildtype strain. Large proportion of vaccine-induced S1-IgG might be non-neutralizing whereas RBD-IgGAM appears to be a good surrogate marker to estimate nAb levels. A third vaccination increases the nAb response. Furthermore, the systemic vaccine does not seem to elicit readily detectable mucosal immunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, et al.. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(1):4704. doi: 10.1038/s41467-020-18450-4 . - DOI - PMC - PubMed
-
- Rockstroh A, Wolf J, Fertey J, Kalbitz S, Schroth S, Lubbert C, et al.. Correlation of humoral immune responses to different SARS-CoV-2 antigens with virus neutralizing antibodies and symptomatic severity in a German COVID-19 cohort. Emerg Microbes Infect. 2021;10(1):774–81. doi: 10.1080/22221751.2021.1913973 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

