Lack of evidence for participation of TMEM150C in sensory mechanotransduction
- PMID: 36256908
- PMCID: PMC9582506
- DOI: 10.1085/jgp.202213098
Lack of evidence for participation of TMEM150C in sensory mechanotransduction
Abstract
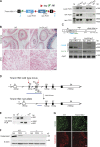
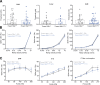
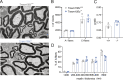
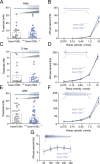
The membrane protein TMEM150C has been proposed to form a mechanosensitive ion channel that is required for normal proprioceptor function. Here, we examined whether expression of TMEM150C in neuroblastoma cells lacking Piezo1 is associated with the appearance of mechanosensitive currents. Using three different modes of mechanical stimuli, indentation, membrane stretch, and substrate deflection, we could not evoke mechanosensitive currents in cells expressing TMEM150C. We next asked if TMEM150C is necessary for the normal mechanosensitivity of cutaneous sensory neurons. We used an available mouse model in which the Tmem150c locus was disrupted through the insertion of a LacZ cassette with a splice acceptor that should lead to transcript truncation. Analysis of these mice indicated that ablation of the Tmem150c gene was not complete in sensory neurons of the dorsal root ganglia (DRG). Using a CRISPR/Cas9 strategy, we made a second mouse model in which a large part of the Tmem150c gene was deleted and established that these Tmem150c-/- mice completely lack TMEM150C protein in the DRGs. We used an ex vivo skin nerve preparation to characterize the mechanosenstivity of mechanoreceptors and nociceptors in the glabrous skin of the Tmem150c-/- mice. We found no quantitative alterations in the physiological properties of any type of cutaneous sensory fiber in Tmem150c-/- mice. Since it has been claimed that TMEM150C is required for normal proprioceptor function, we made a quantitative analysis of locomotion in Tmem150c-/- mice. Here again, we found no indication that there was altered gait in Tmem150c-/- mice compared to wild-type controls. In summary, we conclude that existing mouse models that have been used to investigate TMEM150C function in vivo are problematic. Furthermore, we could find no evidence that TMEM150C forms a mechanosensitive channel or that it is necessary for the normal mechanosensitivity of cutaneous sensory neurons.
© 2022 Ojeda-Alonso et al.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

