Integrative Serum Metabolic Fingerprints Based Multi-Modal Platforms for Lung Adenocarcinoma Early Detection and Pulmonary Nodule Classification
- PMID: 36257825
- PMCID: PMC9731719
- DOI: 10.1002/advs.202203786
Integrative Serum Metabolic Fingerprints Based Multi-Modal Platforms for Lung Adenocarcinoma Early Detection and Pulmonary Nodule Classification
Abstract
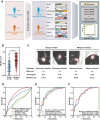
Identification of novel non-invasive biomarkers is critical for the early diagnosis of lung adenocarcinoma (LUAD), especially for the accurate classification of pulmonary nodule. Here, a multiplexed assay is developed on an optimized nanoparticle-based laser desorption/ionization mass spectrometry platform for the sensitive and selective detection of serum metabolic fingerprints (SMFs). Integrative SMFs based multi-modal platforms are constructed for the early detection of LUAD and the classification of pulmonary nodule. The dual modal model, metabolic fingerprints with protein tumor marker neural network (MP-NN), integrating SMFs with protein tumor marker carcinoembryonic antigen (CEA) via deep learning, shows superior performance compared with the single modal model Met-NN (p < 0.001). Based on MP-NN, the tri modal model MPI-RF integrating SMFs, tumor marker CEA, and image features via random forest demonstrates significantly higher performance than the clinical models (Mayo Clinic and Veterans Affairs) and the image artificial intelligence in pulmonary nodule classification (p < 0.001). The developed platforms would be promising tools for LUAD screening and pulmonary nodule management, paving the conceptual and practical foundation for the clinical application of omics tools.
Keywords: deep learning; lung adenocarcinoma; metabolomics; multi-modal; pulmonary nodule.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors have filed patents for both the technology and the use of the technology to analyze biosamples.
Figures



References
-
- Herbst R. S., Morgensztern D., Boshoff C., Nature 2018, 553, 446. - PubMed
-
- Nair A., Bartlett E. C., Walsh S. L. F., Wells A. U., Navani N., Hardavella G., Bhalla S., Calandriello L., Devaraj A., Goo J. M., Klein J. S., MacMahon H., Schaefer‐Prokop C. M., Seo J. B., Sverzellati N., Desai S. R., Eur. Respir. J. 2018, 52, 1801359.
-
- a) Nair V. S., Sundaram V., Desai M., Gould M. K., Am. J. Respir. Crit. Care Med. 2018, 197, 1220; - PMC - PubMed
- b) Massion P. P., Antic S., Ather S., Arteta C., Brabec J., Chen H., Declerck J., Dufek D., Hickes W., Kadir T., Kunst J., Landman B. A., Munden R. F., Novotny P., Peschl H., Pickup L. C., Santos C., Smith G. T., Talwar A., Gleeson F., Am. J. Respir. Crit. Care Med. 2020, 202, 241; - PMC - PubMed
- c) Swensen S. J., Silverstein M. D., Ilstrup D. M., Schleck C. D., Edell E. S., Arch. Intern. Med. 1997, 157, 849; - PubMed
- d) Gould M. K., Ananth L., Barnett P. G., Chest 2007, 131, 383. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81802938/National Natural Science Foundation of China
- 82071990/National Natural Science Foundation of China
- 82001985/National Natural Science Foundation of China
- 81971771/National Natural Science Foundation of China
- 2022YFE0103500/National Key R&D Program of China
- 2021YFF0703500/National Key R&D Program of China
- 2021YFA0910100/National Key R&D Program of China
- 2021-01-07-00-02-E00083/Shanghai Institutions of Higher Learning
- TMSK-2021-124/National Research Center for Translational Medicine Shanghai
- NRCTM(SH)-2021-06/National Research Center for Translational Medicine Shanghai
- SHSMU-ZDCX20210700/Innovative Research Team of High-Level Local Universities in Shanghai
- YG2019QNA44/Medical-Engineering Joint Funds of Shanghai Jiao Tong University
- YG2021ZD09/Medical-Engineering Joint Funds of Shanghai Jiao Tong University
- YG2022QN107/Medical-Engineering Joint Funds of Shanghai Jiao Tong University
- 2019CXJQ03/Innovation Group Project of Shanghai Municipal Health Commission
- SHWRS2020-087/Shanghai "Rising Stars of Medical Talents" Youth Development Program
- CTCCR-2021B06/Clinical Research Innovation Plan of Shanghai General Hospital
- 0206012157/Shanghai General Hospital Characteristic Talent Plan
- 0206012110/Shanghai General Hospital Characteristic Talent Plan
- 19411965200/Project of Shanghai Science and Technology Commission
- 22Y11902800/Project of Shanghai Science and Technology Commission
- 22ZR1450200/Project of Shanghai Science and Technology Commission
- 20ZR1440000/Project of Shanghai Science and Technology Commission
- 20YF1434400/Shanghai Sailing Program
- 19QA1404800/Shanghai Rising-Star Program
LinkOut - more resources
Full Text Sources
Miscellaneous
