Human Islet Amyloid Polypeptide (hIAPP) Protofibril-Specific Antibodies for Detection and Treatment of Type 2 Diabetes
- PMID: 36257905
- PMCID: PMC9731688
- DOI: 10.1002/advs.202202342
Human Islet Amyloid Polypeptide (hIAPP) Protofibril-Specific Antibodies for Detection and Treatment of Type 2 Diabetes
Abstract
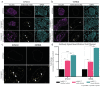
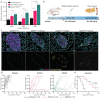
Type 2 diabetes mellitus (T2D) is a major public health concern and is characterized by sustained hyperglycemia due to insulin resistance and destruction of insulin-producing β cells. One pathological hallmark of T2D is the toxic accumulation of human islet amyloid polypeptide (hIAPP) aggregates. Monomeric hIAPP is a hormone normally co-secreted with insulin. However, increased levels of hIAPP in prediabetic and diabetic patients can lead to the formation of hIAPP protofibrils, which are toxic to β cells. Current therapies fail to address hIAPP aggregation and current screening modalities do not detect it. Using a stabilizing capping protein, monoclonal antibodies (mAbs) can be developed against a previously nonisolatable form of hIAPP protofibrils, which are protofibril specific and do not engage monomeric hIAPP. Shown here are two candidate mAbs that can detect hIAPP protofibrils in serum and hIAPP deposits in pancreatic islets in a mouse model of rapidly progressing T2D. Treatment of diabetic mice with the mAbs delays disease progression and dramatically increases overall survival. These results demonstrate the potential for using novel hIAPP protofibril-specific mAbs as a diagnostic screening tool for early detection of T2D, as well as therapeutically to preserve β cell function and target one of the underlying pathological mechanisms of T2D.
Keywords: amyloidosis; diabetes; monoclonal antibodies; therapeutics.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
T.P.S. and W.V.G. are listed as inventors on a US patent related to this study. The authors have no additional financial interests.
Figures




References
-
- International Diabetes Federation , IDF Diabetes Atlas, 10th edn. Brussels, Belgium 2021. https://www.diabetesatlas.org.
-
- Dall T. M., Yang W., Halder P., Pang B., Massoudi M., Wintfeld N., Semilla A. P., Franz J., Hogan P. F., Diabetes Care 2014, 37, 3172. - PubMed
-
- Johnson K. H., O'Brien T. D., Westermark P., Diabetes 1991, 40, 310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- SBIR R43DK125161/GF/NIH HHS/United States
- Mike Hogg Fund
- T32 HL092332/HL/NHLBI NIH HHS/United States
- R01 HD098131/HD/NICHD NIH HHS/United States
- R43DK125161/Small Business Innovation Research
- Eleanor Schwartz Charitable Trust
- Baylor College of Medicine Medical Scientist Training Program
- R01HD099252/HD/NICHD NIH HHS/United States
- Robertson Therapeutic Development Fund
- R01 HD099252/HD/NICHD NIH HHS/United States
- R01HD098131/HD/NICHD NIH HHS/United States
- R43 DK125161/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
