Suppression of heterotopic ossification in fibrodysplasia ossificans progressiva using AAV gene delivery
- PMID: 36258013
- PMCID: PMC9579182
- DOI: 10.1038/s41467-022-33956-9
Suppression of heterotopic ossification in fibrodysplasia ossificans progressiva using AAV gene delivery
Abstract
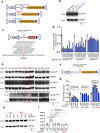
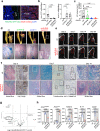
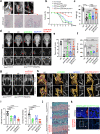
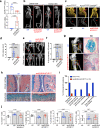
Heterotopic ossification is the most disabling feature of fibrodysplasia ossificans progressiva, an ultra-rare genetic disorder for which there is currently no prevention or treatment. Most patients with this disease harbor a heterozygous activating mutation (c.617 G > A;p.R206H) in ACVR1. Here, we identify recombinant AAV9 as the most effective serotype for transduction of the major cells-of-origin of heterotopic ossification. We use AAV9 delivery for gene replacement by expression of codon-optimized human ACVR1, ACVR1R206H allele-specific silencing by AAV-compatible artificial miRNA and a combination of gene replacement and silencing. In mouse skeletal cells harboring a conditional knock-in allele of human mutant ACVR1 and in patient-derived induced pluripotent stem cells, AAV gene therapy ablated aberrant Activin A signaling and chondrogenic and osteogenic differentiation. In Acvr1(R206H) knock-in mice treated locally in early adulthood or systemically at birth, trauma-induced endochondral bone formation was markedly reduced, while inflammation and fibroproliferative responses remained largely intact in the injured muscle. Remarkably, spontaneous heterotopic ossification also substantially decreased in in Acvr1(R206H) knock-in mice treated systemically at birth or in early adulthood. Collectively, we develop promising gene therapeutics that can prevent disabling heterotopic ossification in mice, supporting clinical translation to patients with fibrodysplasia ossificans progressiva.
© 2022. The Author(s).
Conflict of interest statement
G.G. and J.-H.S. have submitted a patent application concerning the methodology described in this study. G.G. and J.-H.S. are scientific co-founders of AAVAA Therapeutics and hold equity in this company. G.G. is also a scientific co-founder of Voyager Therapeutics and Aspa Therapeutics and holds equity in these companies. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics, Aspa Therapeutics Inc., and other biopharmaceutical companies. E.C.H. serves in a volunteer capacity on the registry advisory board of the IFOPA; on the International Clinical Council on FOP, and on the Fibrous Dysplasia Foundation Medical Advisory Board. E.C.H. received prior research support through his institution from Regeneron Pharmaceuticals. E.C.H. receives clinical trials research support through his institution from Clementia, an Ipsen company. F.S.K. is the founder and past-President of the International Clinical Council (ICC) on FOP. F.S.K. serves in a volunteer capacity on the registry advisory board of the IFOPA. F.S.K. is an investigator on clinical trials sponsored by Clementia, an Ipsen company, and by Regeneron Pharmaceuticals. E.M.S. serves in a volunteer capacity as a research advisor to the IFOPA. These pose no competing interests for this study. Other authors declare no competing interests.
Figures


Similar articles
-
AAV-Mediated Targeting of the Activin A-ACVR1R206H Signaling in Fibrodysplasia Ossificans Progressiva.Biomolecules. 2023 Sep 8;13(9):1364. doi: 10.3390/biom13091364. Biomolecules. 2023. PMID: 37759764 Free PMC article.
-
The ACVR1 R206H mutation found in fibrodysplasia ossificans progressiva increases human induced pluripotent stem cell-derived endothelial cell formation and collagen production through BMP-mediated SMAD1/5/8 signaling.Stem Cell Res Ther. 2016 Aug 17;7(1):115. doi: 10.1186/s13287-016-0372-6. Stem Cell Res Ther. 2016. PMID: 27530160 Free PMC article.
-
Allele-Selective LNA Gapmers for the Treatment of Fibrodysplasia Ossificans Progressiva Knock Down the Pathogenic ACVR1R206H Transcript and Inhibit Osteogenic Differentiation.Nucleic Acid Ther. 2022 Jun;32(3):185-193. doi: 10.1089/nat.2021.0009. Epub 2022 Jan 27. Nucleic Acid Ther. 2022. PMID: 35085461
-
The obligatory role of Activin A in the formation of heterotopic bone in Fibrodysplasia Ossificans Progressiva.Bone. 2018 Apr;109:210-217. doi: 10.1016/j.bone.2017.06.011. Epub 2017 Jun 16. Bone. 2018. PMID: 28629737 Free PMC article. Review.
-
Hints on transcriptional control of essential players in heterotopic ossification of Fibrodysplasia Ossificans Progressiva.Bone. 2018 Apr;109:187-191. doi: 10.1016/j.bone.2017.10.028. Epub 2017 Oct 31. Bone. 2018. PMID: 29100956 Review.
Cited by
-
Navigating the Complex Landscape of Fibrodysplasia Ossificans Progressiva: From Current Paradigms to Therapeutic Frontiers.Genes (Basel). 2023 Nov 30;14(12):2162. doi: 10.3390/genes14122162. Genes (Basel). 2023. PMID: 38136984 Free PMC article. Review.
-
The future of sickle cell disease therapeutics rests in genomics.Dis Model Mech. 2023 Feb 1;16(2):dmm049765. doi: 10.1242/dmm.049765. Epub 2023 Feb 23. Dis Model Mech. 2023. PMID: 36815490 Free PMC article.
-
Immunologic Aspects in Fibrodysplasia Ossificans Progressiva.Biomolecules. 2024 Mar 16;14(3):357. doi: 10.3390/biom14030357. Biomolecules. 2024. PMID: 38540775 Free PMC article. Review.
-
The role of miRNAs as biomarkers in heterotopic ossification.EFORT Open Rev. 2024 Dec 2;9(12):1120-1133. doi: 10.1530/EOR-22-0100. EFORT Open Rev. 2024. PMID: 39620561 Free PMC article. Review.
-
Microfluidic one-step synthesis of a metal-organic framework for osteoarthritis therapeutic microRNAs delivery.Front Bioeng Biotechnol. 2023 Jul 27;11:1239364. doi: 10.3389/fbioe.2023.1239364. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37576986 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

