Controlling Biofilm Development Through Cyclic di-GMP Signaling
- PMID: 36258069
- PMCID: PMC9891824
- DOI: 10.1007/978-3-031-08491-1_3
Controlling Biofilm Development Through Cyclic di-GMP Signaling
Abstract
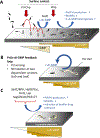
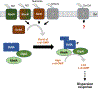
The cyclic di-GMP (c-di-GMP) second messenger represents a signaling system that regulates many bacterial behaviors and is of key importance for driving the lifestyle switch between motile loner cells and biofilm formers. This review provides an up-to-date summary of c-di-GMP pathways connected to biofilm formation by the opportunistic pathogen P. aeruginosa. Emphasis will be on the timing of c-di-GMP production over the course of biofilm formation, to highlight non-uniform and hierarchical increases in c-di-GMP levels, as well as biofilm growth conditions that do not conform with our current model of c-di-GMP.
Keywords: Biofilm; Cyclic di-GMP; Diguanilate cyclase; Nosocomial infection; Phosphodiesterase; Second messenger.
© 2022. The Author(s), under exclusive license to Springer Nature Switzerland AG.
Figures
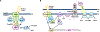


References
-
- CDC. 2019. Antibitic resistance threats in the United States. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019.:1–150.
-
- CDC. 2020. National action plan for combating antibiotic resistant bacteria, 2020–2025.
-
- Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

