The combined value of mpUS and mpMRI-TRUS fusion for the diagnosis of clinically significant prostate cancer
- PMID: 36258247
- PMCID: PMC9580162
- DOI: 10.1186/s40644-022-00498-8
The combined value of mpUS and mpMRI-TRUS fusion for the diagnosis of clinically significant prostate cancer
Abstract
Objective: To evaluate the combined efficacy of multiparametric ultrasonography (mpUS) and multiparametric magnetic resonance imaging/transrectal ultrasound (mpMRI-TRUS) fusion for detecting clinically significant prostate cancer (csPCa).
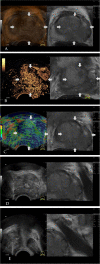
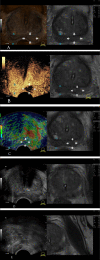
Methods: From November 2019 to September 2021, biopsy-naïve patients underwent mpMRI-TRUS fusion imaging combined with mpUS-guided targeted biopsies (TB) and systematic biopsies (SB). To further evaluate the additional diagnostic value of mpUS, the imaging features of 202 focus obtained from fusion imaging were assessed. The diagnostic accuracies of mpMRI-TRUS fusion imaging and the combination of mpMRI-TRUS fusion imaging with mpUS for csPCa were comparatively evaluated.
Results: A total of 202 prostate lesions (160 patients) were included in the final analysis, of which 105 were csPCa, 16 were ciPCa, and 81 were noncancerous. The median patient age was 69 (65-73) years and the median tPSA was 22.07 (11.22-62.80) ng/mL. For csPCa, the detection rate of TB was higher than that of SB (50.0% vs. 45.5%, p < 0.05). The imaging characteristics of mpUS in the PCa and non-PCa groups were significantly different (p < 0.001). When compared with mpMRI-TRUS fusion imaging, the positive predictive value, false positive rate, and area under the curve (AUC) of csPCa diagnosis by mpMRI-TRUS fusion imaging combined with mpUS increased by 11.30%, decreased by 19.58%, and increased from 0.719 to 0.770 (p < 0.05), respectively.
Conclusion: TB can improve the detection rate of csPCa and hence can be effectively used in the diagnosis and risk assessment of csPCa. The mpUS-enriched valuable diagnostic information for mpMRI-TRUS fusion imaging and their combination showed a higher diagnostic value for csPCa, which can guide subsequent clinical treatment.
Keywords: Contrast-enhanced ultrasonography; Elastography; Multiparametric ultrasound; Prostate cancer; Real-time mpMRI; TRUS fusion biopsy.
© 2022. The Author(s).
Conflict of interest statement
The authors have stated that they have no conflicts of interest.
Figures

Similar articles
-
Prostate cancer detection rate in men undergoing transperineal template-guided saturation and targeted prostate biopsy.Prostate. 2022 Feb;82(3):388-396. doi: 10.1002/pros.24286. Epub 2021 Dec 16. Prostate. 2022. PMID: 34914121 Free PMC article.
-
Targeted multiparametric magnetic resonance imaging/transrectal ultrasound-guided (mpMRI/TRUS) fusion prostate biopsy versus systematic random prostate biopsy: A comparative real-life study.Cancer Rep (Hoboken). 2024 Feb;7(2):e1962. doi: 10.1002/cnr2.1962. Epub 2024 Jan 12. Cancer Rep (Hoboken). 2024. PMID: 38217298 Free PMC article.
-
Diagnostic performance of MRI/TRUS fusion-guided biopsies vs. systematic prostate biopsies in biopsy-naïve, previous negative biopsy patients and men undergoing active surveillance.Minerva Urol Nephrol. 2021 Jun;73(3):357-366. doi: 10.23736/S2724-6051.20.03758-3. Epub 2021 Mar 26. Minerva Urol Nephrol. 2021. PMID: 33769008
-
Multiparametric Magnetic Resonance Imaging in the Diagnosis of Clinically Significant Prostate Cancer: an Updated Systematic Review.Clin Oncol (R Coll Radiol). 2021 Dec;33(12):e599-e612. doi: 10.1016/j.clon.2021.07.016. Epub 2021 Aug 13. Clin Oncol (R Coll Radiol). 2021. PMID: 34400038
-
Elastic Versus Rigid Image Registration in Magnetic Resonance Imaging-transrectal Ultrasound Fusion Prostate Biopsy: A Systematic Review and Meta-analysis.Eur Urol Focus. 2018 Mar;4(2):219-227. doi: 10.1016/j.euf.2016.07.003. Epub 2016 Jul 29. Eur Urol Focus. 2018. PMID: 28753777
Cited by
-
Current Approach to Complications and Difficulties during Transrectal Ultrasound-Guided Prostate Biopsies.J Clin Med. 2024 Jan 16;13(2):487. doi: 10.3390/jcm13020487. J Clin Med. 2024. PMID: 38256621 Free PMC article. Review.
-
Diagnostic accuracy of multiparametric ultrasound in the diagnosis of prostate cancer: systematic review and meta-analysis.Insights Imaging. 2023 Nov 24;14(1):203. doi: 10.1186/s13244-023-01543-1. Insights Imaging. 2023. PMID: 38001351 Free PMC article. Review.
-
Comparative Assessment of Different Ultrasound Technologies in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis.Cancers (Basel). 2023 Aug 15;15(16):4105. doi: 10.3390/cancers15164105. Cancers (Basel). 2023. PMID: 37627133 Free PMC article. Review.
References
-
- Borghesi M, Bianchi L, Barbaresi U, Vagnoni V, Corcioni B , Gaudiano C, et al. Diagnostic performance of MRI/TRUS fusion-guided biopsies vs. systematic prostate biopsies in biopsy-naïve, previous negative biopsy patients and men undergoing active surveillance. Minerva Urol Nephrol. 2021;73(3):357–366. doi: 10.23736/S2724-6051.20.03758-3. - DOI - PubMed
-
- Del Monte M, Cipollari S, Del Giudice F, Pecoraro M, Bicchetti M, Messina E, et al. MRI-directed biopsy for primary detection of prostate cancer in a population of 223 men: MRI In-Bore vs MRI-transrectal ultrasound fusion-targeted techniques. Br J Radiol. 2022;95(1131):20210528. doi: 10.1259/bjr.20210528. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

