Demystifying racemic natural products in the homochiral world
- PMID: 36259059
- PMCID: PMC9562063
- DOI: 10.1038/s41570-022-00431-4
Demystifying racemic natural products in the homochiral world
Abstract
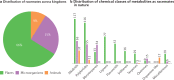
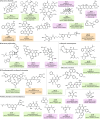
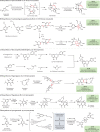
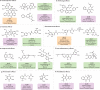
Natural products possess structural complexity, diversity and chirality with attractive functions and biological activities that have significantly impacted drug discovery initiatives. Chiral natural products are abundant in nature but rarely occur as racemates. The occurrence of natural products as racemates is very intriguing from a biosynthetic point of view; as enzymes are chiral molecules, enzymatic reactions generating natural products should be stereospecific and lead to single-enantiomer products. Despite several reports in the literature describing racemic mixtures of stereoisomers isolated from natural sources, there has not been a comprehensive review of these intriguing racemic natural products. The discovery of many more natural racemates and their potential enzymatic sources in recent years allows us to describe the distribution and chemical diversity of this 'class of natural products' to enrich discussions on biosynthesis. In this Review, we describe the chemical classes, occurrence and distribution of pairs of enantiomers in nature and provide insights about recent advances in analytical methods used for their characterization. Special emphasis is on the biosynthesis, including plausible enzymatic and non-enzymatic formation of natural racemates, and their pharmacological significance.
Keywords: Biosynthesis; Natural products; Pharmacology; Structure elucidation.
© Springer Nature Limited 2022, Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures

References
-
- Sharma B. Nature of chiral drugs and their occurrence in environment. J. Xenobiotics. 2014;4:14–19. doi: 10.4081/xeno.2014.2272. - DOI
-
- Devínsky F. Chirality and the origin of life. Symmetry. 2021;13:2277. doi: 10.3390/sym13122277. - DOI
-
- Kittakoop P. Part 2: occurrence of racemic natural products and their biological activities. J. Chulabhorn R. Acad. 2020;2:27–43.
Publication types
LinkOut - more resources
Full Text Sources

