Ectopic foci do not co-locate with ventricular epicardial stretch during early acute regional ischemia in isolated pig hearts
- PMID: 36259098
- PMCID: PMC9579492
- DOI: 10.14814/phy2.15492
Ectopic foci do not co-locate with ventricular epicardial stretch during early acute regional ischemia in isolated pig hearts
Abstract
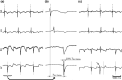
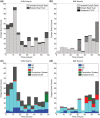
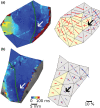
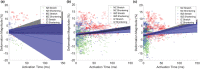
Ectopic activation during early acute regional ischemia may initiate fatal reentrant arrhythmias. However, the origin of this ectopy remains poorly understood. Studies suggest that systolic stretch arising from dyskinesia in ischemic tissue may cause ectopic depolarization due to cardiac mechanosensitivity. The aim of this study was to investigate the link between mechanical stretch and ectopic electrical activation during early acute regional ischemia. We used a recently developed optical mapping technique capable of simultaneous imaging of mechanical deformation and electrical activation in isolated hearts. Eight domestic swine hearts were prepared in left ventricular working mode (LVW), in which the left ventricle was loaded and contracting. In an additional eight non-working (NW) hearts, contraction was pharmacologically suppressed with blebbistatin and the left ventricle was not loaded. In both groups, the left anterior descending coronary artery was tied below the first diagonal branch. Positive mechanical stretch (bulging) during systole was observed in the ischemic zones of LVW, but not NW, hearts. During ischemia phase 1a (0-15 min post-occlusion), LVW hearts had more ectopic beats than NW hearts (median: 19, interquartile range: 10-28 vs. median: 2, interquartile range: 1-6; p = 0.02); but the difference during phase 1b (15-60 min post-occlusion) was not significant (median: 27, interquartile range: 22-42 vs. median: 16, interquartile range: 12-31; p = 0.37). Ectopic beats arose preferentially from the ischemic border zone in both groups (p < 0.01). In LVW hearts, local mechanical stretch was only occasionally co-located with ectopic foci (9 of 69 ectopic beats). Despite the higher rate of ectopy observed in LVW hearts during ischemia phase 1a, the ectopic beats generally did not arise by the hypothesized mechanism in which ectopic foci are generated by co-local epicardial mechanical stretch.
© 2022 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declares that there is no conflict of interest.
Figures




References
-
- Akaishi, M. , Weintraub, W. S. , Schneider, R. M. , Klein, L. W. , Agarwal, J. B. , & Helfant, R. H. (1986). Analysis of systolic bulging. Mechanical characteristics of acutely ischemic myocardium in the conscious dog. Circulation Research, 58, 209–217. - PubMed
-
- Allen, D. G. , & Orchard, C. H. (1987). Myocardial contractile function during ischemia and hypoxia. Circulation Research, 60, 153–168. - PubMed
-
- Bachtel, A. D. , Gray, R. A. , Stohlman, J. M. , Bourgeois, E. B. , Pollard, A. E. , & Rogers, J. M. (2011). A novel approach to dual excitation ratiometric optical mapping of cardiac action potentials with di‐4‐ANEPPS using pulsed LED excitation. IEEE Transactions on Biomedical Engineering, 58, 2120–2126. - PMC - PubMed
-
- Bode, F. , Sachs, F. , & Franz, M. R. (2001). Tarantula peptide inhibits atrial fibrillation. Nature, 409, 35–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

