Chlorogenic acid alleviates the reduction of Akt and Bad phosphorylation and of phospho-Bad and 14-3-3 binding in an animal model of stroke
- PMID: 36259103
- PMCID: PMC9715392
- DOI: 10.4142/jvs.22200
Chlorogenic acid alleviates the reduction of Akt and Bad phosphorylation and of phospho-Bad and 14-3-3 binding in an animal model of stroke
Abstract
Background: Stroke is caused by disruption of blood supply and results in permanent disabilities as well as death. Chlorogenic acid is a phenolic compound found in various fruits and coffee and exerts antioxidant, anti-inflammatory, and anti-apoptotic effects.
Objectives: The purpose of this study was to investigate whether chlorogenic acid regulates the PI3K-Akt-Bad signaling pathway in middle cerebral artery occlusion (MCAO)-induced damage.
Methods: Chlorogenic acid (30 mg/kg) or vehicle was administered peritoneally to adult male rats 2 h after MCAO surgery, and animals were sacrificed 24 h after MCAO surgery. Neurobehavioral tests were performed, and brain tissues were isolated. The cerebral cortex was collected for Western blot and immunoprecipitation analyses.
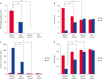
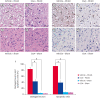
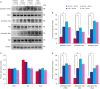
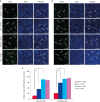
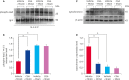
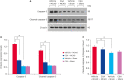
Results: MCAO damage caused severe neurobehavioral disorders and chlorogenic acid improved the neurological disorders. Chlorogenic acid alleviated the MCAO-induced histopathological changes and decreased the number of terminal deoxynucleotidyl transferase dUTP nick end labeling-positive cells. Furthermore, MCAO-induced damage reduced the expression of phospho-PDK1, phospho-Akt, and phospho-Bad, which was alleviated with administration of chlorogenic acid. The interaction between phospho-Bad and 14-3-3 levels was reduced in MCAO animals, which was attenuated by chlorogenic acid treatment. In addition, chlorogenic acid alleviated the increase of cytochrome c and caspase-3 expression caused by MCAO damage.
Conclusions: The results of the present study showed that chlorogenic acid activates phospho-Akt and phospho-Bad and promotes the interaction between phospho-Bad and 14-3-3 during MCAO damage. In conclusion, chlorogenic acid exerts neuroprotective effects by activating the Akt-Bad signaling pathway and maintaining the interaction between phospho-Bad and 14-3-3 in ischemic stroke model.
Keywords: Chlorogenic acid; ischemic stroke; neuroprotection.
© 2022 The Korean Society of Veterinary Science.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Retinoic acid alleviates the reduction of Akt and Bad phosphorylation and regulates Bcl-2 family protein interactions in animal models of ischemic stroke.PLoS One. 2024 May 16;19(5):e0303213. doi: 10.1371/journal.pone.0303213. eCollection 2024. PLoS One. 2024. PMID: 38753710 Free PMC article.
-
Chlorogenic acid alleviates neurobehavioral disorders and brain damage in focal ischemia animal models.Neurosci Lett. 2021 Aug 24;760:136085. doi: 10.1016/j.neulet.2021.136085. Epub 2021 Jun 24. Neurosci Lett. 2021. PMID: 34174343
-
Neuroprotective effects of chlorogenic acid by controlling the Bcl-2 family protein in a stroke animal model.J Vet Med Sci. 2023 Jun 20. doi: 10.1292/jvms.23-0153. Online ahead of print. J Vet Med Sci. 2023. PMID: 37344388
-
Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation.Neurosci Lett. 2012 Jan 24;507(2):156-60. doi: 10.1016/j.neulet.2011.12.012. Epub 2011 Dec 17. Neurosci Lett. 2012. PMID: 22200499
-
Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine.Curr Neuropharmacol. 2017;15(4):471-479. doi: 10.2174/1570159X14666160325120625. Curr Neuropharmacol. 2017. PMID: 27012954 Free PMC article. Review.
Cited by
-
Identification of proteins regulated by chlorogenic acid in an ischemic animal model: a proteomic approach.Lab Anim Res. 2023 Jun 5;39(1):12. doi: 10.1186/s42826-023-00164-5. Lab Anim Res. 2023. PMID: 37271817 Free PMC article.
-
Apitherapy in Post-Ischemic Brain Neurodegeneration of Alzheimer's Disease Proteinopathy: Focus on Honey and Its Flavonoids and Phenolic Acids.Molecules. 2023 Jul 25;28(15):5624. doi: 10.3390/molecules28155624. Molecules. 2023. PMID: 37570596 Free PMC article. Review.
References
-
- Ingall T. Stroke--incidence, mortality, morbidity and risk. J Insur Med. 2004;36(2):143–152. - PubMed
-
- Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871–895. - PubMed
-
- Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38(7):1167–1186. - PubMed
-
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–e339. - PubMed
-
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem Res. 2004;29(11):1943–1949. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

