Rheology of marine sponges reveals anisotropic mechanics and tuned dynamics
- PMID: 36259170
- PMCID: PMC9579767
- DOI: 10.1098/rsif.2022.0476
Rheology of marine sponges reveals anisotropic mechanics and tuned dynamics
Abstract
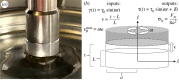
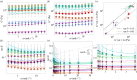
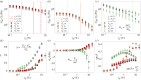
Sponges are animals that inhabit many aquatic environments while filtering small particles and ejecting metabolic wastes. They are composed of cells in a bulk extracellular matrix, often with an embedded scaffolding of stiff, siliceous spicules. We hypothesize that the mechanical response of this heterogeneous tissue to hydrodynamic flow influences cell proliferation in a manner that generates the body of a sponge. Towards a more complete picture of the emergence of sponge morphology, we dissected a set of species and subjected discs of living tissue to physiological shear and uniaxial deformations on a rheometer. Various species exhibited rheological properties such as anisotropic elasticity, shear softening and compression stiffening, negative normal stress, and non-monotonic dissipation as a function of both shear strain and frequency. Erect sponges possessed aligned, spicule-reinforced fibres which endowed three times greater stiffness axially compared with orthogonally. By contrast, tissue taken from shorter sponges was more isotropic but time-dependent, suggesting higher flow sensitivity in these compared with erect forms. We explore ecological and physiological implications of our results and speculate about flow-induced mechanical signalling in sponge cells.
Keywords: anisotropic elasticity; auxeticity; marine sponges; nonlinear viscoelasticity; rheology; tissue mechanics.
Figures






References
-
- Garrone R. 1978. Phylogenesis of connective tissue. Basel, Switzerland: Karger Publishers.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
