Changes in MCP-1, HGF, and IGF-1 expression in endometrial stromal cells, PBMCs, and PFMCs of endometriotic women following 1,25(OH)2D3 treatment
- PMID: 36259314
- PMCID: PMC9667513
- DOI: 10.1111/jcmm.17592
Changes in MCP-1, HGF, and IGF-1 expression in endometrial stromal cells, PBMCs, and PFMCs of endometriotic women following 1,25(OH)2D3 treatment
Abstract
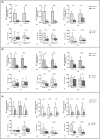
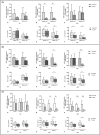
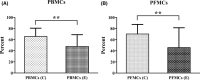
1,25(OH)2D3 has anti-inflammatory and growth inhibitory effects. Our study explored the effect of 1,25(OH)2D3 treatment on the expression of monocyte chemotactic protein-1 (MCP-1), hepatocyte growth factor (HGF), and insulin-like growth factor-1 (IGF-1) by peripheral blood mononuclear cells (PBMCs), peritoneal fluid mononuclear cells (PFMCs), endometrial stromal cells (ESCs), and its effect on the proliferation of PBMCs and PFMCs of patients with endometriosis compared with controls. PBMCs, PFMCs, and ESCs were obtained from 10 endometriosis patients and 10 non-endometriotic individuals. After treating cells with 0.1 μM of 1,25(OH)2D3 for 6, 24, and 48 h, the gene and protein expression of mentioned factors were evaluated by real-time PCR and ELISA methods, respectively. 1,25(OH)2D3 treatment significantly reduced the protein expression of MCP-1, HGF, and IGF-1 in PBMCs and PFMCs of endometriotic patients at 48 h (p < 0.05-<0.01). Also, this treatment significantly reduced MCP-1, HGF, and IGF-1 gene and/or protein expression in EESCs and EuESCs at 24 and 48 h (p < 0.05-<0.01). 1,25(OH)2D3 treatment also reduced the proliferation of PBMCs and PFMCs of endometriotic patients compared with controls (p < 0.01). 1,25(OH)2D3 can be considered as a potentially effective agent in the prevention and treatment of endometriosis along with other therapies.
Keywords: 1,25(OH)2D3; HGF; IGF-1; MCP-1; endometrial stromal cells; endometriosis; mononuclear cells.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures

Similar articles
-
Expression levels of MCP-1, HGF, and IGF-1 in endometriotic patients compared with non-endometriotic controls.BMC Womens Health. 2021 Dec 20;21(1):422. doi: 10.1186/s12905-021-01560-6. BMC Womens Health. 2021. PMID: 34930225 Free PMC article.
-
Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women.Phytother Res. 2019 Apr;33(4):1044-1054. doi: 10.1002/ptr.6298. Epub 2019 Mar 6. Phytother Res. 2019. PMID: 30838714
-
Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls.BMC Womens Health. 2020 Jan 6;20(1):3. doi: 10.1186/s12905-019-0865-4. BMC Womens Health. 2020. PMID: 31906916 Free PMC article.
-
Resveratrol treatment reduces expression of MCP-1, IL-6, IL-8 and RANTES in endometriotic stromal cells.J Cell Mol Med. 2021 Jan;25(2):1116-1127. doi: 10.1111/jcmm.16178. Epub 2020 Dec 15. J Cell Mol Med. 2021. PMID: 33325132 Free PMC article.
-
Intercellular adhesion molecule-1 and hepatocyte growth factor in human endometriosis: original investigation and a review of literature.Gynecol Obstet Invest. 1999;47 Suppl 1:11-6; discussion 16-7. doi: 10.1159/000052854. Gynecol Obstet Invest. 1999. PMID: 10087423 Review.
Cited by
-
Decoding the Role of Insulin-like Growth Factor 1 and Its Isoforms in Breast Cancer.Int J Mol Sci. 2024 Aug 27;25(17):9302. doi: 10.3390/ijms25179302. Int J Mol Sci. 2024. PMID: 39273251 Free PMC article. Review.
-
Vitamin D and reproductive disorders: a comprehensive review with a focus on endometriosis.Reprod Health. 2024 May 2;21(1):61. doi: 10.1186/s12978-024-01797-y. Reprod Health. 2024. PMID: 38698459 Free PMC article. Review.
References
-
- Shafrir AL, Farland LV, Shah DK, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1‐15. - PubMed
-
- Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422‐469.
-
- Symons LK, Miller JE, Kay VR, et al. The immunopathophysiology of endometriosis. Trends Mol Med. 2018;24(9):748‐762. - PubMed
-
- Jorgensen H, Hill AS, Beste MT, et al. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil Steril. 2017;107(5):1191‐1199 e2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

