Limited Weight Impact After Switching From Boosted Protease Inhibitors to Dolutegravir in Persons With Human Immunodeficiency Virus With High Cardiovascular Risk: A Post Hoc Analysis of the 96-Week NEAT-022 Randomized Trial
- PMID: 36259527
- PMCID: PMC7617433
- DOI: 10.1093/cid/ciac827
Limited Weight Impact After Switching From Boosted Protease Inhibitors to Dolutegravir in Persons With Human Immunodeficiency Virus With High Cardiovascular Risk: A Post Hoc Analysis of the 96-Week NEAT-022 Randomized Trial
Abstract
Background: In the NEAT022 trial, virologically suppressed persons with human immunodeficiency virus (HIV) at high cardiovascular risk switching from protease inhibitors to dolutegravir either immediately (DTG-I) or after 48 weeks (DTG-D) showed noninferior virological suppression and significant lipid and cardiovascular disease risk reductions on switching to dolutegravir relative to continuing protease inhibitors.
Methods: In post hoc analysis, major endpoints were 48-week and 96-week weight and body mass index (BMI) changes. Factors associated with weight/BMI changes within the first 48 weeks of DTG exposure, proportion of participants by category of percentage weight change, proportions of BMI categories over time, and impact on metabolic outcomes were also assessed.
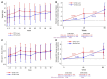
Results: Between May 2014 and November 2015, 204 (DTG-I) and 208 (DTG-D) participants were included. Weight significantly increased (mean, +0.810 kg DTG-I arm, and +0.979 kg DTG-D arm) in the first 48 weeks postswitch, but remained stable from 48 to 96 weeks in DTG-I arm. Switching from darunavir, White race, total to high-density lipoprotein cholesterol ratio <3.7, and normal/underweight BMI were independently associated with higher weight/BMI gains. The proportion of participants with ≥5% weight change increased similarly in both arms over time. The proportions of BMI categories, use of lipid-lowering drugs, diabetes and/or use of antidiabetic agents, and hypertension and/or use of antihypertensive agents did not change within or between arms at 48 and 96 weeks.
Conclusions: Switching from protease inhibitors to dolutegravir in persons with HIV with high cardiovascular risk led to modest weight gain limited to the first 48 weeks, which involved preferentially normal-weight or underweight persons and was not associated with negative metabolic outcomes.
Clinical trials registration: NCT02098837 and EudraCT 2013-003704-39.
Keywords: dolutegravir; switch; weight.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. L. W. has received consulting fees, honoraria for lectures, advisory boards, or travel grants from Gilead, Janssen, MSD, and ViiV. A. G.-C. has received honoraria for lectures, advisory boards, or travel grants and her institution has received research grants from Gilead, Janssen, MSD, and ViiV. S. R. has received grants or contracts, honoraria for lectures, advisory boards, or travel grants from Gilead, Janssen, MSD, Theratechnologies, and ViiV. P. D. has received honoraria for lectures or advisory boards and his institution has received research grants from Gilead, Janssen, MSD, and ViiV Healthcare, and consulting fees from Gilead Sciences, ViiV Healthcare, Janssen-Cilag, and MSD. M. G. has received consulting fees, honoraria for lectures, advisory boards, or travel grants from Gilead, MSD, and ViiV. F. R. has received consulting fees, honoraria for lectures, advisory boards, or travel grants from Gilead, Janssen, MSD, Theratechnologies, and ViiV, and grants or contracts from MSD. C. S. has received consulting fees, honoraria for lectures, advisory boards, or travel grants from Gilead, Janssen, and MSD. M. M. has received consulting fees, honoraria for lectures, advisory boards or travel grants from ViiV, Janssen, and MSD. J. R. has received consulting fees (paid to author) and honoraria for lectures, advisory boards, or travel grants from Abivax, Boehringer, Galapagos, Gilead, Janssen, Merck Theratechnologies, and ViiV, including participation on a data safety monitoring board or advisory board from Abivax and Galapagos (paid to author) and leadership or fiduciary role in other board, society, committee or advocacy group from the European AIDS Clinical Society (EACS) (unpaid participation). C. K. has received consulting fees, honoraria for lectures, advisory boards, or travel grants and her institution has received research grants from Gilead, MSD, and ViiV. G. M. N. B. has received honoraria for lectures, advisory boards, or travel grants and his institution has received research grants from Gilead, Janssen, MSD, and ViiV, including leadership or fiduciary role in other board, society, committee or advocacy group for Chair of the EACS Treatment Recommendations. G. M. has received consulting fees, honoraria for lectures, advisory boards, or travel grants and his institution has received research grants from Gilead, MSD, Theratechnologies, and ViiV. J. F. has received consulting fees, honoraria for lectures, advisory boards, or travel grants from Gilead, Janssen, MSD, and ViiV. H. J. S. has received honoraria for lectures, advisory boards, or travel grants and his institution has received research grants from Gilead, GSK, Heidelberg Immunotherapeutics, Janssen, MSD, and ViiV, including consulting fees from Gilead Sciences, ViiV Health Care, MSD, and Janssen-Cilag (paid to author); participation on a data safety monitoring board or advisory board for Gilead Sciences, ViiV Healthcare, and MSD; leadership or fiduciary role in other board, society, committee, or advocacy group for the EACS guideline working group, German-Austrian guideline working group, and Federal Off-Label Commission German AIDS Society Chairmanship (in the past); and receipt of equipment materials, drugs, medical writing, gifts or other services from Gilead Sciences. G. G. has received honoraria for lectures, advisory boards, or travel grants and participation on a data safety monitoring board or advisory board; his institution has received research grants from Gilead, MSD, and ViiV. E. F. has received honoraria for lectures, advisory boards, or travel grants and received grants or contracts from Gilead, Janssen, MSD, and ViiV (paid to institution). S. E. has received consulting fees, honoraria for lectures, advisory boards, or travel grants and research grants or contracts from Gilead, MSD, and ViiV. J. M. G. is a full-time employee of and owns stock in ViiV as Senior Global Medical Director since 1 May 2018. A. P. has received consulting fees and honoraria for lectures or advisory boards and his institution has received research grants from Gilead, Janssen, MSD, and ViiV. E. M. has received consulting fees and honoraria for lectures or advisory boards and his institution has received research grants from Gilead, Janssen, MSD, Theratechnologies, and ViiV. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Gatell JM, Assoumou L, Moyle G, et al. Immediate versus deferred switching from a boosted protease inhibitor–based regimen to a dolutegravir-based regimen in virologically suppressed patients with high cardiovascular risk or age ≥50 years: final 96-week results of the NEAT022 study. Clin Infect Dis. 2018;68:597–606. - PubMed
-
- Bansi-Matharu L, Phillips A, Oprea C, et al. Contemporary antiretrovirals and body-mass index: a prospective study of the RESPOND cohort consortium. Lancet HIV. 2021;8:e711–22. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

