Effectiveness of mRNA Booster Vaccination Against Mild, Moderate, and Severe COVID-19 Caused by the Omicron Variant in a Large, Population-Based, Norwegian Cohort
- PMID: 36259543
- PMCID: PMC9620770
- DOI: 10.1093/infdis/jiac419
Effectiveness of mRNA Booster Vaccination Against Mild, Moderate, and Severe COVID-19 Caused by the Omicron Variant in a Large, Population-Based, Norwegian Cohort
Abstract
Background: Understanding how booster vaccination can prevent moderate and severe illness without hospitalization is crucial to evaluate the full advantage of mRNA boosters.
Methods: We followed 85 801 participants (aged 31-81 years) in 2 large population-based cohorts during the Omicron BA.1/2 wave. Information on home testing, PCR testing, and symptoms of coronavirus disease 2019 (COVID-19) was extracted from biweekly questionnaires covering the period 12 January 2022 to 7 April 2022. Vaccination status and data on previous SARS-CoV-2 infection were obtained from national registries. Cox regression was used to estimate the effectiveness of booster vaccination compared to receipt of 2-dose primary series >130 days previously.
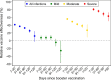
Results: The effectiveness of booster vaccination increased with increasing severity of COVID-19 and decreased with time since booster vaccination. The effectiveness against severe COVID-19 was reduced from 80.9% shortly after booster vaccination to 63.4% in the period >90 days after vaccination. There was hardly any effect against mild COVID-19. The effectiveness tended to be lower among subjects aged ≥60 years than those aged <50 years.
Conclusions: This is the first population-based study to evaluate booster effectiveness against self-reported mild, moderate, and severe COVID-19. Our findings contribute valuable information on duration of protection and thus timing of additional booster vaccinations.
Keywords: COVID-19; MoBa; SARS-CoV-2; booster vaccination; disease severity; mRNA vaccine; vaccine effectiveness.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interests. J. S. has contributed to vaccine effectiveness studies funded by the European Medicines Agency and the European Centre for Disease Prevention and Control. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Similar articles
-
Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study.Lancet Infect Dis. 2023 Apr;23(4):421-434. doi: 10.1016/S1473-3099(22)00732-0. Epub 2022 Dec 12. Lancet Infect Dis. 2023. PMID: 36521506 Free PMC article.
-
Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar.N Engl J Med. 2022 May 12;386(19):1804-1816. doi: 10.1056/NEJMoa2200797. Epub 2022 Mar 9. N Engl J Med. 2022. PMID: 35263534 Free PMC article.
-
Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial.Lancet Infect Dis. 2024 Apr;24(4):351-360. doi: 10.1016/S1473-3099(23)00650-3. Epub 2023 Dec 20. Lancet Infect Dis. 2024. PMID: 38141632 Clinical Trial.
-
Facing the Omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review.Front Immunol. 2022 Aug 24;13:940562. doi: 10.3389/fimmu.2022.940562. eCollection 2022. Front Immunol. 2022. PMID: 36091023 Free PMC article.
-
Real-world evidence on the efficacy of bivalent booster doses of SARS-CoV-2 vaccine in respect of monovalent boosters or primary cycle of vaccination: a narrative review.Epidemiol Prev. 2023 Nov-Dec;47(6):331-343. doi: 10.19191/EP23.6.A626.081. Epidemiol Prev. 2023. PMID: 38314543 Review. English.
Cited by
-
Effectiveness of full (booster) COVID-19 vaccination against severe outcomes and work absenteeism in hospitalized patients with COVID-19 during the Delta and Omicron waves in Greece.Vaccine. 2023 Mar 31;41(14):2343-2348. doi: 10.1016/j.vaccine.2023.01.067. Epub 2023 Feb 2. Vaccine. 2023. PMID: 36740558 Free PMC article.
-
Effect of Vaccination against SARS-CoV-2 on Long COVID-19: A Narrative Review.Life (Basel). 2022 Dec 8;12(12):2057. doi: 10.3390/life12122057. Life (Basel). 2022. PMID: 36556422 Free PMC article. Review.
-
Incidence and Determinants of Symptomatic and Asymptomatic SARS-CoV-2 Breakthrough Infections After Booster Dose in a Large European Multicentric Cohort of Health Workers-ORCHESTRA Project.J Epidemiol Glob Health. 2023 Sep;13(3):577-588. doi: 10.1007/s44197-023-00139-8. Epub 2023 Jul 22. J Epidemiol Glob Health. 2023. PMID: 37480426 Free PMC article.
-
Determinants of humoral and cellular immune responses to three doses of mRNA SARS-CoV-2 vaccines in older adults: a longitudinal cohort study.Lancet Healthy Longev. 2023 May;4(5):e188-e199. doi: 10.1016/S2666-7568(23)00055-7. Lancet Healthy Longev. 2023. PMID: 37148891 Free PMC article.
-
Estimating the trend of COVID-19 in Norway by combining multiple surveillance indicators.PLoS One. 2025 Jan 30;20(1):e0317105. doi: 10.1371/journal.pone.0317105. eCollection 2025. PLoS One. 2025. PMID: 39883654 Free PMC article.
References
-
- World Health Organization . Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern.https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1..... Accessed 6 July 2022.
-
- Norwegian Institute of Public Health . Weekly reports for coronavirus and COVID-19. Week 2.https://www.fhi.no/contentassets/8a971e7b0a3c4a06bdbf381ab52e6157/vedleg.... Accessed 5 July 2022.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

