A Dynamic rRNA Ribomethylome Drives Stemness in Acute Myeloid Leukemia
- PMID: 36259929
- PMCID: PMC9900322
- DOI: 10.1158/2159-8290.CD-22-0210
A Dynamic rRNA Ribomethylome Drives Stemness in Acute Myeloid Leukemia
Abstract
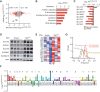
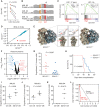
The development and regulation of malignant self-renewal remain unresolved issues. Here, we provide biochemical, genetic, and functional evidence that dynamics in ribosomal RNA (rRNA) 2'-O-methylation regulate leukemia stem cell (LSC) activity in vivo. A comprehensive analysis of the rRNA 2'-O-methylation landscape of 94 patients with acute myeloid leukemia (AML) revealed dynamic 2'-O-methylation specifically at exterior sites of ribosomes. The rRNA 2'-O-methylation pattern is closely associated with AML development stage and LSC gene expression signature. Forced expression of the 2'-O-methyltransferase fibrillarin (FBL) induced an AML stem cell phenotype and enabled engraftment of non-LSC leukemia cells in NSG mice. Enhanced 2'-O-methylation redirected the ribosome translation program toward amino acid transporter mRNAs enriched in optimal codons and subsequently increased intracellular amino acid levels. Methylation at the single site 18S-guanosine 1447 was instrumental for LSC activity. Collectively, our work demonstrates that dynamic 2'-O-methylation at specific sites on rRNAs shifts translational preferences and controls AML LSC self-renewal.
Significance: We establish the complete rRNA 2'-O-methylation landscape in human AML. Plasticity of rRNA 2'-O-methylation shifts protein translation toward an LSC phenotype. This dynamic process constitutes a novel concept of how cancers reprogram cell fate and function. This article is highlighted in the In This Issue feature, p. 247.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
References
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730–7. - PubMed
-
- Saba JA, Liakath-Ali K, Green R, Watt FM. Translational control of stem cell function. Nat Rev Mol Cell Biol 2021;22:671–90. - PubMed
-
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. . Global quantification of mammalian gene expression control. Nature 2011;473:337–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

