The diagnosis, classification, and treatment of sarcoma in this era of artificial intelligence and immunotherapy
- PMID: 36260064
- PMCID: PMC9759765
- DOI: 10.1002/cac2.12373
The diagnosis, classification, and treatment of sarcoma in this era of artificial intelligence and immunotherapy
Abstract
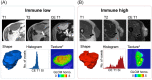
Soft-tissue sarcomas (STS) represent a group of rare and heterogeneous tumors associated with several challenges, including incorrect or late diagnosis, the lack of clinical expertise, and limited therapeutic options. Digital pathology and radiomics represent transformative technologies that appear promising for improving the accuracy of cancer diagnosis, characterization and monitoring. Herein, we review the potential role of the application of digital pathology and radiomics in managing patients with STS. We have particularly described the main results and the limits of the studies using radiomics to refine diagnosis or predict the outcome of patients with soft-tissue sarcomas. We also discussed the current limitation of implementing radiomics in routine settings. Standard management approaches for STS have not improved since the early 1970s. Immunotherapy has revolutionized cancer treatment; nonetheless, immuno-oncology agents have not yet been approved for patients with STS. However, several lines of evidence indicate that immunotherapy may represent an efficient therapeutic strategy for this group of diseases. Thus, we emphasized the remarkable potential of immunotherapy in sarcoma treatment by focusing on recent data regarding the immune landscape of these tumors. We have particularly emphasized the fact that the development of immunotherapy for sarcomas is not an aspect of histology (except for alveolar soft-part sarcoma) but rather that of the tumor microenvironment. Future studies investigating immunotherapy strategies in sarcomas should incorporate at least the presence of tertiary lymphoid structures as a stratification factor in their design, besides including a strong translational program that will allow for a better understanding of the determinants involved in sensitivity and treatment resistance to immune-oncology agents.
Keywords: artificial intelligence; digital pathology; immunotherapy; radiomics; sarcoma.
© 2022 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
AI: Research grant: AstraZeneca, Bayer, BMS Merck, MSD, and Roche. Advisory Board: AstraZeneca, Bayer, Janssen Cilag, Lilly, Roche, Blueprint medicines, and PharmaMar. AC: No competing interests.
Figures
References
-
- Italiano A, Mathoulin‐Pelissier S, Cesne AL, Terrier P, Bonvalot S, Collin F, et al. Trends in survival for patients with metastatic soft‐tissue sarcoma. Cancer. 2011;117(5):1049‐1054. - PubMed
-
- Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta‐analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft‐tissue sarcoma. Cancer. 2008;113(3):573‐581. - PubMed
-
- Italiano A, Le Cesne A, Mendiboure J, Blay J‐Y, Piperno‐Neumann S, Chevreau C, et al. Prognostic factors and impact of adjuvant treatments on local and metastatic relapse of soft‐tissue sarcoma patients in the competing risks setting. Cancer. 2014;120(21):3361‐3369. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

