Unmasking a two-faced protein
- PMID: 36260068
- PMCID: PMC9581526
- DOI: 10.7554/eLife.83482
Unmasking a two-faced protein
Abstract
Single-molecule fluorescence spectroscopy and molecular dynamics simulations illuminate the structure and dynamics of PSD-95, a protein involved in neural plasticity.
Keywords: E. coli; molecular biophysics; neuroscience; postsynaptic density; protein dynamics; single molecule fluorescence; single-molecule FRET; structural biology.
© 2022, Maslov and Hendrix.
Conflict of interest statement
IM, JH No competing interests declared
Figures
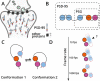
Comment on
-
Fuzzy supertertiary interactions within PSD-95 enable ligand binding.Elife. 2022 Sep 7;11:e77242. doi: 10.7554/eLife.77242. Elife. 2022. PMID: 36069777 Free PMC article.
References
-
- Fernández E, Collins MO, Uren RT, Kopanitsa MV, Komiyama NH, Croning MDR, Zografos L, Armstrong JD, Choudhary JS, Grant SGN. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Molecular Systems Biology. 2009;5:269. doi: 10.1038/msb.2009.27. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

