Clinical practice guidelines for duodenal cancer 2021
- PMID: 36260172
- PMCID: PMC9663352
- DOI: 10.1007/s00535-022-01919-y
Clinical practice guidelines for duodenal cancer 2021
Abstract
Duodenal cancer is considered to be a small intestinal carcinoma in terms of clinicopathology. In Japan, there are no established treatment guidelines based on sufficient scientific evidence; therefore, in daily clinical practice, treatment is based on the experience of individual physicians. However, with advances in diagnostic modalities, it is anticipated that opportunities for its detection will increase in future. We developed guidelines for duodenal cancer because this disease is considered to have a high medical need from both healthcare providers and patients for appropriate management. These guidelines were developed for use in actual clinical practice for patients suspected of having non-ampullary duodenal epithelial malignancy and for patients diagnosed with non-ampullary duodenal epithelial malignancy. In this study, a practice algorithm was developed in accordance with the Minds Practice Guideline Development Manual 2017, and Clinical Questions were set for each area of epidemiology and diagnosis, endoscopic treatment, surgical treatment, and chemotherapy. A draft recommendation was developed through a literature search and systematic review, followed by a vote on the recommendations. We made decisions based on actual clinical practice such that the level of evidence would not be the sole determinant of the recommendation. This guideline is the most standard guideline as of the time of preparation. It is important to decide how to handle each case in consultation with patients and their family, the treating physician, and other medical personnel, considering the actual situation at the facility (and the characteristics of the patient).
Keywords: Clinical practice guidelines; Duodenal cancer; The Japan duodenal cancer guideline committee; Treatment.
© 2022. The Author(s).
Conflict of interest statement
Any financial relationship with enterprises, businesses, or academic institutions in the subject matter or materials discussed in the manuscript are listed as follows: MSD, Ono Pharmaceutical, Medical Beebe Partners, 3D Matrix, Nihon Pharmaceuticals, Fujifilm, Olympus, Tella Pharma, ICON Japan, IQVIA Services Japan, Gunze Medical Japan, Janssen Pharma, Kyorin Pharmaceutical, Covidien Japan, Sanofi, Nippon Kayaku, Johnson & Johnson, Nichi-Iko Pharmaceutical, Central Medical, Terumo, HOYA Pentax, Mitsubishi Tanabe Pharma, Tsumura & Co., EA Pharma, Astellas Pharma, Eisai, Shionogi & Co., Daiichi Sankyo, Taiho Pharmaceutical, Takeda Pharmaceutical, Chugai Pharmaceutical, Bayer Yakuhin, Yakult Honsha, AbbVie, Astra Zeneca, Eli Lilly Japan, Pfizer, Gilead Sciences, Merck Serono, Sumitomo Dainippon Pharma Co., Mediscience Planning Inc., Solasia Pharma, Parexel International Inc., and Amgen Astellas BioPharma.
Figures


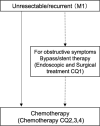
References
-
- Kojimahara N, Nakayama T, Morizane T, et al. Manual for Minds Clinical Practice Guideline Development 2017, Japan Council for Quality Health Care. https://minds.jcqhc.or.jp/s/developer_manual(in Japanese).
-
- Bojesen R, Andersson M, Riis L, et al. Incidence of phenotypes of and survival from small bowel cancer in Denmark, 1994–2010: a population-based study. J Gastroenterol. 2016;51:891–899. - PubMed
-
- Legué L, Bernards N, Gerritse S, et al. Trends in incidence, treatment, and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in the Netherlands. Acta Oncol. 2016;55:1183–1189. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

