A Smart Shoe Insole to Monitor Frail Older Adults' Walking Speed: Results of Two Evaluation Phases Completed in a Living Lab and Through a 12-Week Pilot Study
- PMID: 36260404
- PMCID: PMC8406107
- DOI: 10.2196/15641
A Smart Shoe Insole to Monitor Frail Older Adults' Walking Speed: Results of Two Evaluation Phases Completed in a Living Lab and Through a 12-Week Pilot Study
Abstract
Background: Recent World Health Organization reports propose wearable devices to collect information on activity and walking speed as innovative health indicators. However, mainstream consumer-grade tracking devices and smartphone apps are often inaccurate and require long-term acceptability assessment.
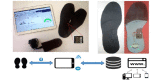
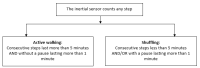
Objective: Our aim is to assess the user acceptability of an instrumented shoe insole in frail older adults. This device monitors participants' walking speed and differentiates active walking from shuffling after step length calibration.
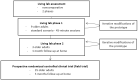
Methods: A multiphase evaluation has been designed: 9 older adults were evaluated in a living lab for a day, 3 older adults were evaluated at home for a month, and a prospective randomized trial included 35 older adults at home for 3 months. A qualitative research design using face-to-face and phone semistructured interviews was performed. Our hypothesis was that this shoe insole was acceptable in monitoring long-term outdoor and indoor walking. The primary outcome was participants' acceptability, measured by a qualitative questionnaire and average time of insole wearing per day. The secondary outcome described physical frailty evolution in both groups.
Results: Living lab results confirmed the importance of a multiphase design study with participant involvement. Participants proposed insole modifications. Overall acceptability had mixed results: low scores for reliability (2.1 out of 6) and high scores for usability (4.3 out of 6) outcomes. The calibration phase raised no particular concern. During the field test, a majority of participants (mean age 79 years) were very (10/16) or quite satisfied (3/16) with the insole's comfort at the end of the follow-up. Participant insole acceptability evolved as follows: 63% (12/19) at 1 month, 50% (9/18) at 2 months, and 75% (12/16) at 3 months. A total of 9 participants in the intervention group discontinued the intervention because of technical issues. All participants equipped for more than a week reported wearing the insole every day at 1 month, 83% (15/18) at 2 months, and 94% (15/16) at 3 months for 5.8, 6.3, and 5.1 hours per day, respectively. Insole data confirmed that participants effectively wore the insole without significant decline during follow-up for an average of 13.5 days per 4 months and 5.6 hours per day. For secondary end points, the change in frailty parameters or quality of life did not differ for those randomly assigned to the intervention group compared to usual care.
Conclusions: Our study reports acceptability data on an instrumented insole in indoor and outdoor walking with remote monitoring in frail older adults under real-life conditions. To date, there is limited data in this population set. This thin instrumentation, including a flexible battery, was a technical challenge and seems to provide an acceptable solution over time that is valued by participants. However, users still raised certain acceptability issues. Given the growing interest in wearable health care devices, these results will be useful for future developments.
Trial registration: ClinicalTrials.gov NCT02316600; https://clinicaltrials.gov/ct2/show/NCT02316600.
Keywords: activity tracker; frail older adults; outpatient monitoring; shoe insert; walking speed.
©Antoine Piau, Zara Steinmeyer, Yoann Charlon, Laetitia Courbet, Vincent Rialle, Benoit Lepage, Eric Campo, Fati Nourhashemi. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org), 05.07.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
A Smart Insole to Promote Healthy Aging for Frail Elderly Individuals: Specifications, Design, and Preliminary Results.JMIR Rehabil Assist Technol. 2015 May 25;2(1):e5. doi: 10.2196/rehab.4084. JMIR Rehabil Assist Technol. 2015. PMID: 28582238 Free PMC article.
-
Application of smart bracelet to monitor frailty-related gait parameters of older Chinese adults: A preliminary study.Geriatr Gerontol Int. 2018 Sep;18(9):1366-1371. doi: 10.1111/ggi.13492. Epub 2018 Aug 14. Geriatr Gerontol Int. 2018. PMID: 30105810
-
The frail-LESS (LEss sitting and sarcopenia in frail older adults) remote intervention to improve sarcopenia and maintain independent living via reductions in sedentary behaviour: findings from a randomised controlled feasibility trial.BMC Geriatr. 2024 Sep 9;24(1):747. doi: 10.1186/s12877-024-05310-9. BMC Geriatr. 2024. PMID: 39251904 Free PMC article. Clinical Trial.
-
Shoe-Insole Technology for Injury Prevention in Walking.Sensors (Basel). 2018 May 8;18(5):1468. doi: 10.3390/s18051468. Sensors (Basel). 2018. PMID: 29738486 Free PMC article. Review.
-
Insole-Based Systems for Health Monitoring: Current Solutions and Research Challenges.Sensors (Basel). 2022 Jan 7;22(2):438. doi: 10.3390/s22020438. Sensors (Basel). 2022. PMID: 35062398 Free PMC article. Review.
Cited by
-
Cross-sectional study comparing smart insoles and manual methods for short physical performance battery in hip fracture patients.Aging Clin Exp Res. 2025 Mar 1;37(1):61. doi: 10.1007/s40520-025-02960-6. Aging Clin Exp Res. 2025. PMID: 40021544 Free PMC article.
-
The most used questionnaires for evaluating the usability of robots and smart wearables: A scoping review.Digit Health. 2024 Apr 9;10:20552076241237384. doi: 10.1177/20552076241237384. eCollection 2024 Jan-Dec. Digit Health. 2024. PMID: 38601185 Free PMC article.
-
Validation of the Short Physical Performance Battery via Plantar Pressure Analysis Using Commercial Smart Insoles.Sensors (Basel). 2023 Dec 11;23(24):9757. doi: 10.3390/s23249757. Sensors (Basel). 2023. PMID: 38139603 Free PMC article.
-
Attributes, Methods, and Frameworks Used to Evaluate Wearables and Their Companion mHealth Apps: Scoping Review.JMIR Mhealth Uhealth. 2024 Apr 5;12:e52179. doi: 10.2196/52179. JMIR Mhealth Uhealth. 2024. PMID: 38578671 Free PMC article.
-
Random forest algorithms to classify frailty and falling history in seniors using plantar pressure measurement insoles: a large-scale feasibility study.BMC Geriatr. 2022 Sep 12;22(1):746. doi: 10.1186/s12877-022-03425-5. BMC Geriatr. 2022. PMID: 36096722 Free PMC article.
References
-
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. - DOI - PubMed
-
- Metzelthin SF, van Rossum E, de Witte LP, Ambergen AW, Hobma SO, Sipers W, Kempen GIJM. Effectiveness of interdisciplinary primary care approach to reduce disability in community dwelling frail older people: cluster randomised controlled trial. BMJ. 2013 Sep 10;347:f5264. doi: 10.1136/bmj.f5264. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=24022033 - DOI - PMC - PubMed
-
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, King AC, Kritchevsky SB, Manini TM, McDermott MM, Miller ME, Newman AB, Rejeski WJ, Sink KM, Williamson JD, LIFE study investigators Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014 Jun 18;311(23):2387–96. doi: 10.1001/jama.2014.5616. http://europepmc.org/abstract/MED/24866862 - DOI - PMC - PubMed
-
- Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel J, Lloyd-Sherlock P, Epping-Jordan JE, Peeters GMEEG, Mahanani WR, Thiyagarajan JA, Chatterji S. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016 May 21;387(10033):2145–2154. doi: 10.1016/S0140-6736(15)00516-4. http://europepmc.org/abstract/MED/26520231 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

