VISTA Targeting of T-cell Quiescence and Myeloid Suppression Overcomes Adaptive Resistance
- PMID: 36260656
- PMCID: PMC10544831
- DOI: 10.1158/2326-6066.CIR-22-0116
VISTA Targeting of T-cell Quiescence and Myeloid Suppression Overcomes Adaptive Resistance
Abstract
V domain immunoglobulin suppressor of T-cell activation (VISTA) is a premier target for cancer treatment due to its broad expression in many cancer types and enhanced expression upon development of adaptive immune checkpoint resistance. In the CT26 colorectal cancer model, monotherapy of small tumors with anti-VISTA resulted in slowed tumor growth. In a combination therapy setting, large CT26 tumors showed complete adaptive resistance to anti-PD-1/CTLA-4, but inclusion of anti-VISTA led to rejection of half the tumors. Mechanisms of enhanced antitumor immunity were investigated using single-cell RNA sequencing (scRNA-seq), multiplex image analysis, and flow cytometry of the tumor immune infiltrate. In both treatment models, anti-VISTA upregulated stimulated antigen presentation pathways and reduced myeloid-mediated suppression. Imaging revealed an anti-VISTA stimulated increase in contacts between T cells and myeloid cells, further supporting the notion of increased antigen presentation. scRNA-seq of tumor-specific CD8+ T cells revealed that anti-VISTA therapy induced T-cell pathways highly distinct from and complementary to those induced by anti-PD-1 therapy. Whereas anti-CTLA-4/PD-1 expanded progenitor exhausted CD8+ T-cell subsets, anti-VISTA promoted costimulatory genes and reduced regulators of T-cell quiescence. Notably, this is the first report of a checkpoint regulator impacting CD8+ T-cell quiescence, and the first indication that quiescence may be a target in the context of T-cell exhaustion and in cancer. This study builds a foundation for all future studies on the role of anti-VISTA in the development of antitumor immunity and provides important mechanistic insights that strongly support use of anti-VISTA to overcome the adaptive resistance seen in contemporary treatments involving PD-1 and/or CTLA-4. See related Spotlight by Wei, p. 3.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures
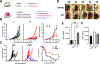


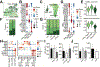



Comment in
-
Surveying Mechanisms of Immune Checkpoint Blockade from a VISTA.Cancer Immunol Res. 2023 Jan 3;11(1):3. doi: 10.1158/2326-6066.CIR-22-0708. Cancer Immunol Res. 2023. PMID: 36318053
Comment on
-
Surveying Mechanisms of Immune Checkpoint Blockade from a VISTA.Cancer Immunol Res. 2023 Jan 3;11(1):3. doi: 10.1158/2326-6066.CIR-22-0708. Cancer Immunol Res. 2023. PMID: 36318053
References
-
- Turajlic S, et al., Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. The Lancet Oncology, 2017. 18(8): p. 1009––1021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

