Activity of a direct VTA to ventral pallidum GABA pathway encodes unconditioned reward value and sustains motivation for reward
- PMID: 36260661
- PMCID: PMC9581470
- DOI: 10.1126/sciadv.abm5217
Activity of a direct VTA to ventral pallidum GABA pathway encodes unconditioned reward value and sustains motivation for reward
Abstract
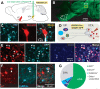
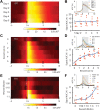
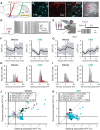
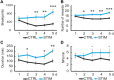
Dopamine signaling from the ventral tegmental area (VTA) plays critical roles in reward-related behaviors, but less is known about the functions of neighboring VTA GABAergic neurons. We show here that a primary target of VTA GABA projection neurons is the ventral pallidum (VP). Activity of VTA-to-VP-projecting GABA neurons correlates consistently with size and palatability of the reward and does not change following cue learning, providing a direct measure of reward value. Chemogenetic stimulation of this GABA projection increased activity of a subset of VP neurons that were active while mice were seeking reward. Optogenetic stimulation of this pathway improved performance in a cue-reward task and maintained motivation to work for reward over days. This VTA GABA projection provides information about reward value directly to the VP, likely distinct from the prediction error signal carried by VTA dopamine neurons.
Figures



Comment in
-
Invariant inhibition to calculate prediction errors?Trends Neurosci. 2023 Apr;46(4):257-259. doi: 10.1016/j.tins.2023.01.004. Epub 2023 Jan 26. Trends Neurosci. 2023. PMID: 36707259
References
-
- Morales M., Margolis E. B., Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85 (2017). - PubMed
-
- Schultz W., Dayan P., Montague P. R., A neural substrate of prediction and reward. Science 275, 1593–1599 (1997). - PubMed
-
- Wise R. A., Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 (2004). - PubMed
-
- Tolu S., Eddine R., Marti F., David V., Graupner M., Pons S., Baudonnat M., Husson M., Besson M., Reperant C., Zemdegs J., Pagès C., Hay Y. A. H., Lambolez B., Caboche J., Gutkin B., Gardier A. M., Changeux J. P., Faure P., Maskos U., Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol. Psychiatry 18, 382–393 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

