Numb regulates Tau levels and prevents neurodegeneration in tauopathy mouse models
- PMID: 36260685
- PMCID: PMC9581485
- DOI: 10.1126/sciadv.abm4295
Numb regulates Tau levels and prevents neurodegeneration in tauopathy mouse models
Abstract
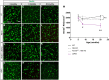
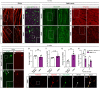
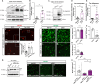
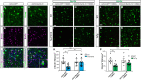
Accumulation of the microtubule-associated protein Tau is linked to neuronal cell death in tauopathies, but how intraneuronal Tau levels are regulated in health and disease remains unclear. Here, we show that conditional inactivation of the trafficking adaptor protein Numb in retinal ganglion cells (RGCs) increases Tau levels and leads to axonal blebbing, which is followed by neuronal cell loss in aged mice. In the TauP301S mouse model of tauopathy, conditional inactivation of Numb in RGCs and spinal motoneurons accelerates neurodegeneration, and loss of Numb in motoneurons also leads to precocious hindlimb paralysis. Conversely, overexpression of the long isoform of Numb (Numb-72) decreases intracellular Tau levels and reduces axonal blebbing in TauP301S RGCs, leading to improved electrical activity in cultured neurons and improves performance in a visually guided behavior test in vivo. These results uncover Numb as a key regulator of intracellular Tau levels and identify Numb-72 as a potential therapeutic factor for tauopathies.
Figures




Comment in
-
NUMB72 - a therapeutic target for tauopathies?Nat Rev Neurol. 2022 Dec;18(12):699. doi: 10.1038/s41582-022-00745-3. Nat Rev Neurol. 2022. PMID: 36323923 No abstract available.
References
-
- Qiang L., Sun X., Austin T. O., Muralidharan H., Jean D. C., Liu M., Yu W., Baas P. W., Tau does not stabilize axonal microtubules but rather enables them to have long labile domains. Curr. Biol. 28, 2181–2189.e4 (2018). - PubMed
-
- Watanabe H., Bagarinao E., Yokoi T., Yamaguchi H., Ishigaki S., Mausuda M., Katsuno M., Sobue G., Tau accumulation and network breakdown in Alzheimer’s disease. Adv. Exp. Med. Biol. 1184, 231–240 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

