Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: a meta-analysis of 128 patients
- PMID: 36260735
- PMCID: PMC9813524
- DOI: 10.1182/bloodadvances.2022008525
Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: a meta-analysis of 128 patients
Abstract
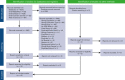
Relapsed/refractory primary central nervous system lymphoma (PCNSL) and secondary central nervous system lymphoma (SCNSL) are associated with short survival and represent an unmet need, requiring novel effective strategies. Anti-CD19 chimeric antigen receptor (CAR) T cells, effective in systemic large B-cell lymphoma (LBCL), have shown responses in PCNSL and SCNSL in early reports, but with limited sample size. We, therefore, performed a comprehensive systematic review and meta-analysis of all published data describing CAR T-cell use in PCNSL and SCNSL. This identified 128 patients with PCNSL (30) and SCNSL (98). Our primary objectives were to evaluate CAR T-cell specific toxicity (immune effector cell-associated neurotoxicity syndrome [ICANS] and cytokine release syndrome [CRS]) as well as response rates in these 2 populations. Seventy percent of patients with PCNSL had CRS of any grade (13% grade 3-4) and 53% had ICANS of any grade (18% grade 3-4). Comparatively, 72% of the SCNSL cohort experienced CRS of any grade (11% grade 3-4) and 48% had ICANS of any grade (26% grade 3-4). Of the patients with PCNSL, 56% achieved a complete remission (CR) with 37% remaining in remission at 6 months. Similarly, 47% of patients with SCNSL had a CR, with 37% in remission at 6 months. In a large meta-analysis of central nervous system (CNS) lymphomas, toxicity of anti-CD19-CAR T-cell therapy was similar to that of registrational studies in systemic LBCL with no increased signal of neurotoxicity observed. Encouraging efficacy was demonstrated in patients with CNS lymphoma with no discernible differences between PCNSL and SCNSL.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: K.D. served on the advisory board/consulting for AstraZeneca, Beigene, AbbVie, Daiichi Sankyo, ADC Therapeutics, Incyte, Morphosys, Genmab. P.N.M. served on the advisory board for Incyte; consultancy: Incyte; speakers bureau: Incyte, Kite Pharma. A.G. acted in a consulting or advisory role for AstraZeneca, Bristol Myers Squibb, Celgene, Hoffmann-La Roche, Janssen, Kite, Morphosys, Allopex, Gilead, Novartis, Vincerx, Resilience, received research funding (institutional) from Acerta, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Hoffmann-La Roche, Infinity, Janssen, Karyopharm, Kite, Morphosys, Pharmacyclics, Seattle Genetics, Verastem. A.S. served as a consultant (Kite/Gilead, Magenta Therapeutics, Incyte Pharmaceuticals, CareDx) and received royalty fees from In8Bio Inc. A.A. served on the advisory board/consulting for Incyte. The remaining authors declare no competing financial interests.
Figures


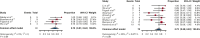
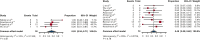
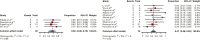
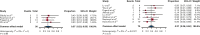
References
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2018;380(1):45–56. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed
-
- Green K, Hogg JP. StatPearls. StatPearls Publishing LLC; 2022. Central Nervous System Lymphoma. - PubMed
-
- Jahnke K, Thiel E, Martus P, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80(2):159–165. - PubMed

