The L-NAME mouse model of preeclampsia and impact to long-term maternal cardiovascular health
- PMID: 36260752
- PMCID: PMC9356384
- DOI: 10.26508/lsa.202201517
The L-NAME mouse model of preeclampsia and impact to long-term maternal cardiovascular health
Abstract
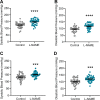
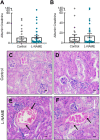
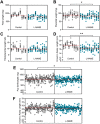
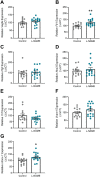
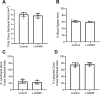
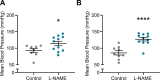
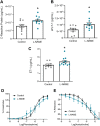
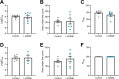
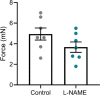
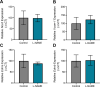
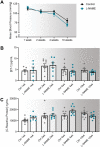
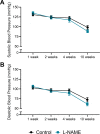
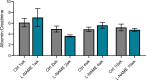
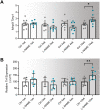
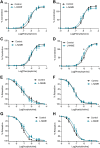
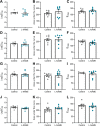
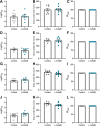
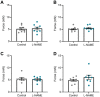
Preeclampsia affects ∼2-8% of pregnancies worldwide. It is associated with increased long-term maternal cardiovascular disease risk. This study assesses the effect of the vasoconstrictor N(ω)-nitro-L-arginine methyl ester (L-NAME) in modelling preeclampsia in mice, and its long-term effects on maternal cardiovascular health. In this study, we found that L-NAME administration mimicked key characteristics of preeclampsia, including elevated blood pressure, impaired fetal and placental growth, and increased circulating endothelin-1 (vasoconstrictor), soluble fms-like tyrosine kinase-1 (anti-angiogenic factor), and C-reactive protein (inflammatory marker). Post-delivery, mice that received L-NAME in pregnancy recovered, with no discernible changes in measured cardiovascular indices at 1-, 2-, and 4-wk post-delivery, compared with matched controls. At 10-wk post-delivery, arteries collected from the L-NAME mice constricted significantly more to phenylephrine than controls. In addition, these mice had increased kidney Mmp9:Timp1 and heart Tnf mRNA expression, indicating increased inflammation. These findings suggest that though administration of L-NAME in mice certainly models key characteristics of preeclampsia during pregnancy, it does not appear to model the adverse increase in cardiovascular disease risk seen in individuals after preeclampsia.
© 2022 de Alwis et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Celastrol attenuates symptoms of preeclampsia in rats by inhibiting matrix metalloproteinase-9.Eur J Pharmacol. 2018 Jul 5;830:33-38. doi: 10.1016/j.ejphar.2018.04.025. Epub 2018 Apr 22. Eur J Pharmacol. 2018. PMID: 29694830
-
Preeclampsia associated changes in volume density of fetoplacental vessels in Chinese women and mouse model of preeclampsia.Placenta. 2022 Apr;121:116-125. doi: 10.1016/j.placenta.2022.03.002. Epub 2022 Mar 9. Placenta. 2022. PMID: 35306432
-
Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia.J Clin Invest. 2016 Jul 1;126(7):2561-74. doi: 10.1172/JCI83918. Epub 2016 Jun 6. J Clin Invest. 2016. PMID: 27270170 Free PMC article.
-
An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array.Am J Obstet Gynecol. 2022 Feb;226(2S):S963-S972. doi: 10.1016/j.ajog.2020.10.023. Epub 2021 Mar 9. Am J Obstet Gynecol. 2022. PMID: 33712272 Review.
-
Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia.Pediatr Res. 2005 May;57(5 Pt 2):1R-7R. doi: 10.1203/01.PDR.0000159567.85157.B7. Epub 2005 Apr 6. Pediatr Res. 2005. PMID: 15817508 Review.
Cited by
-
Scutellariae Radix and Atractylodis Macrocephalae Rhizoma pairs ameliorate preeclampsia via PI3K/AKT/eNOS pathway.Front Pharmacol. 2025 Jul 11;16:1614167. doi: 10.3389/fphar.2025.1614167. eCollection 2025. Front Pharmacol. 2025. PMID: 40717981 Free PMC article.
-
Neurodevelopmental Disruptions in Children of Preeclamptic Mothers: Pathophysiological Mechanisms and Consequences.Int J Mol Sci. 2024 Mar 24;25(7):3632. doi: 10.3390/ijms25073632. Int J Mol Sci. 2024. PMID: 38612445 Free PMC article. Review.
-
Reliability of Rodent and Rabbit Models in Preeclampsia Research.Int J Mol Sci. 2022 Nov 18;23(22):14344. doi: 10.3390/ijms232214344. Int J Mol Sci. 2022. PMID: 36430816 Free PMC article. Review.
-
Investigating the Impact of Maternal Obesity on Disease Severity in a Mouse Model of Preeclampsia.Nutrients. 2025 May 5;17(9):1586. doi: 10.3390/nu17091586. Nutrients. 2025. PMID: 40362895 Free PMC article.
-
Extracellular Vesicles Derived from Human Umbilical Cord MSC Improve Vascular Endothelial Function in In Vitro and In Vivo Models of Preeclampsia through Activating Arginine Metabolism.Mol Pharm. 2023 Dec 4;20(12):6429-6440. doi: 10.1021/acs.molpharmaceut.3c00816. Epub 2023 Oct 30. Mol Pharm. 2023. PMID: 37903292 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
