Ionic Liquids as Biocompatible Antibacterial Agents: A Case Study on Structure-Related Bioactivity on Escherichia coli
- PMID: 36260814
- PMCID: PMC9778738
- DOI: 10.1021/acsabm.2c00615
Ionic Liquids as Biocompatible Antibacterial Agents: A Case Study on Structure-Related Bioactivity on Escherichia coli
Abstract
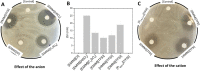
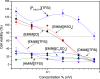
The potential of ionic liquids (ILs) to be used as antimicrobial agents for biomedical applications has been hindered by the fact that most of them are cytotoxic toward mammalian cells. Understanding the mechanism of bacterial and mammalian cellular damage of ILs is key to their safety design. In this work, we evaluate the antimicrobial activity and mode of action of several ILs with varying anions and cations toward the clinically relevant Gram-negative Escherichia coli. Langmuir monolayer technique was used to evaluate if the IL's mode of action was related to the bacterial cell membrane interaction for an effective E. coli killing. 1-Decyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide [DMIM][TFSI] and trihexyltetradecyl phosphonium bis(trifluoromethylsulfonyl) imide [P6,6,6,14][TFSI] were surface-active and induced bacterial cell lysis, through a membrane-disruption phenomenon on bacteria, in a mechanism that was clearly related to the long alkyl chains of the cation. 1-Ethyl-3-methylimidazolium hydrogen sulfate [EMIM][HSO4] was highly antimicrobial toward E. coli and found suitable for biological applications since it was harmless to mammalian cells at most of the tested concentrations. The results suggest that the imidazolium cation of the ILs is mostly responsible not only for their antimicrobial activity but also for their cytotoxicity, and the inclusion of different anions may tailor the ILs' biocompatibility without losing the capacity to kill bacteria, as is the case of [EMIM][HSO4]. Importantly, this IL was found to be highly antimicrobial even when incorporated in a polymeric matrix.
Keywords: Escherichia coli; antimicrobial; biocompatible; ionic liquids; surface activity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Synthesis, Characterization, Biological Evaluation, and In Silico Studies of Imidazolium-, Pyridinium-, and Ammonium-Based Ionic Liquids Containing n-Butyl Side Chains.Molecules. 2022 Oct 6;27(19):6650. doi: 10.3390/molecules27196650. Molecules. 2022. PMID: 36235187 Free PMC article.
-
Toxicity and inhibition assessment of ionic liquids by activated sludge.Ecotoxicol Environ Saf. 2020 Jan 15;187:109836. doi: 10.1016/j.ecoenv.2019.109836. Epub 2019 Oct 30. Ecotoxicol Environ Saf. 2020. PMID: 31675504
-
Antimicrobial properties of novel ionic liquids derived from imidazolium cation with phenolic functional groups.Bioorg Chem. 2021 Oct;115:105289. doi: 10.1016/j.bioorg.2021.105289. Epub 2021 Aug 20. Bioorg Chem. 2021. PMID: 34426154
-
Recent advances in the application of ionic liquids in antimicrobial material for air disinfection and sterilization.Front Cell Infect Microbiol. 2023 May 17;13:1186117. doi: 10.3389/fcimb.2023.1186117. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37265495 Free PMC article. Review.
-
Ionic Liquids: Emerging Antimicrobial Agents.Pharm Res. 2022 Oct;39(10):2391-2404. doi: 10.1007/s11095-022-03336-5. Epub 2022 Jul 25. Pharm Res. 2022. PMID: 35879499 Review.
Cited by
-
From Synthesis to Functionality: Tailored Ionic Liquid-Based Electrospun Fibers with Superior Antimicrobial Properties.Polymers (Basel). 2024 Jul 23;16(15):2094. doi: 10.3390/polym16152094. Polymers (Basel). 2024. PMID: 39125121 Free PMC article.
-
Beyond One-Size-Fits-All: Addressing Methodological Constraints in Novel Antimicrobials Discovery.Antibiotics (Basel). 2025 Aug 21;14(8):848. doi: 10.3390/antibiotics14080848. Antibiotics (Basel). 2025. PMID: 40868042 Free PMC article.
-
Biocompatible Liquid Embolic for the Treatment of Microvascular Hemorrhage.Adv Sci (Weinh). 2024 Sep;11(34):e2403615. doi: 10.1002/advs.202403615. Epub 2024 Jul 25. Adv Sci (Weinh). 2024. PMID: 39049735 Free PMC article.
-
Chiral Salen-Based Organic Salts: Synthesis and Potential Antibacterial Activity.Molecules. 2025 May 15;30(10):2173. doi: 10.3390/molecules30102173. Molecules. 2025. PMID: 40430345 Free PMC article.
-
Selective Blood Cell Hitchhiking in Whole Blood with Ionic Liquid-Coated PLGA Nanoparticles to Redirect Biodistribution After Intravenous Injection.Res Sq [Preprint]. 2023 Jul 11:rs.3.rs-3146716. doi: 10.21203/rs.3.rs-3146716/v1. Res Sq. 2023. PMID: 37502854 Free PMC article. Preprint.
References
-
- Correia D. M.; Fernandes L. C.; Martins P. M.; García-Astrain C.; Costa C. M.; Reguera J.; Lanceros-Méndez S.. Ionic Liquid–Polymer Composites: A New Platform for Multifunctional Applications. 2020, 301909736. 10.1002/adfm.201909736. - DOI
-
- Kurnia K. A.; Sintra T. E.; Neves C. M.; Shimizu K.; Canongia Lopes J. N.; Gonçalves F.; Ventura S. P.; Freire M. G.; Santos L. M.; Coutinho J. A. The effect of the cation alkyl chain branching on mutual solubilities with water and toxicities. Phys. Chem. Chem. Phys. 2014, 16, 19952–19963. 10.1039/C4CP02309A. - DOI - PMC - PubMed
-
- Yu G.; Zhao D.; Wen L.; Yang S.; Chen X. Viscosity of ionic liquids: Database, observation, and quantitative structure-property relationship analysis. AIChE J. 2012, 58, 2885–2899. 10.1002/aic.12786. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

