Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211)
- PMID: 36260828
- PMCID: PMC9995105
- DOI: 10.1200/JCO.22.01013
Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211)
Abstract
Purpose: Patients with advanced pancreatic neuroendocrine tumors (NETs) have few treatment options that yield objective responses. Retrospective and small prospective studies suggest that capecitabine and temozolomide are associated with high response rates (RRs) and long progression-free survival (PFS).
Patients and methods: E2211 was a multicenter, randomized, phase II trial comparing temozolomide versus capecitabine/temozolomide in patients with advanced low-grade or intermediate-grade pancreatic NETs. Key eligibility criteria included progression within the preceding 12 months and no prior temozolomide, dimethyl-triazeno-imidazole-carboxamide or dacarbazine, capecitabine or fluorouracil. The primary end point was PFS; secondary endpoints were overall survival, RR, safety, and methylguanine methyltransferase (MGMT) by immunohistochemistry and promoter methylation.
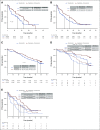
Results: A total of 144 patients were enrolled between April 2013 and March 2016 to temozolomide (n = 72) or capecitabine and temozolomide (n = 72); the primary analysis population included 133 eligible patients. At the scheduled interim analysis in January 2018, the median PFS was 14.4 months for temozolomide versus 22.7 months for capecitabine/temozolomide (hazard ratio = 0.58), which was sufficient to reject the null hypothesis for the primary end point (stratified log-rank P = .022). In the final analysis (May 2021), the median overall survival was 53.8 months for temozolomide and 58.7 months for capecitabine/temozolomide (hazard ratio = 0.82, P = .42). MGMT deficiency was associated with response.
Conclusion: The combination of capecitabine/temozolomide was associated with a significant improvement in PFS compared with temozolomide alone in patients with advanced pancreatic NETs. The median PFS and RR observed with capecitabine/temozolomide are the highest reported in a randomized study for pancreatic NETs. MGMT deficiency was associated with response, and although routine MGMT testing is not recommended, it can be considered for select patients in need of objective response (ClinicalTrials.gov identifier: NCT01824875).
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Raymond E, Dahan L, Raoul JL, et al. : Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364:501-513, 2011 - PubMed
-
- Brabander T, van der Zwan WA, Teunissen JJM, et al. : Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 23:4617-4624, 2017 - PubMed
-
- Moertel CG, Hanley JA, Johnson LA: Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med 303:1189-1194, 1980 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233320/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- UG1 CA233160/CA/NCI NIH HHS/United States
- UG1 CA189863/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- UG1 CA233253/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- UG1 CA189821/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233337/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

