NRG Oncology/RTOG1205: A Randomized Phase II Trial of Concurrent Bevacizumab and Reirradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma
- PMID: 36260832
- PMCID: PMC9940937
- DOI: 10.1200/JCO.22.00164
NRG Oncology/RTOG1205: A Randomized Phase II Trial of Concurrent Bevacizumab and Reirradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma
Abstract
Purpose: To assess whether reirradiation (re-RT) and concurrent bevacizumab (BEV) improve overall survival (OS) and/or progression-free survival (PFS), compared with BEV alone in recurrent glioblastoma (GBM). The primary objective was OS, and secondary objectives included PFS, response rate, and treatment adverse events (AEs) including delayed CNS toxicities.
Methods: NRG Oncology/RTOG1205 is a prospective, phase II, randomized trial of re-RT and BEV versus BEV alone. Stratification factors included age, resection, and Karnofsky performance status (KPS). Patients with recurrent GBM with imaging evidence of tumor progression ≥ 6 months from completion of prior chemo-RT were eligible. Patients were randomly assigned 1:1 to re-RT, 35 Gy in 10 fractions, with concurrent BEV IV 10 mg/kg once in every 2 weeks or BEV alone until progression.
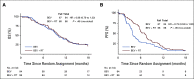
Results: From December 2012 to April 2016, 182 patients were randomly assigned, of whom 170 were eligible. Patient characteristics were well balanced between arms. The median follow-up for censored patients was 12.8 months. There was no improvement in OS for BEV + RT, hazard ratio, 0.98; 80% CI, 0.79 to 1.23; P = .46; the median survival time was 10.1 versus 9.7 months for BEV + RT versus BEV alone. The median PFS for BEV + RT was 7.1 versus 3.8 months for BEV, hazard ratio, 0.73; 95% CI, 0.53 to 1.0; P = .05. The 6-month PFS rate improved from 29.1% (95% CI, 19.1 to 39.1) for BEV to 54.3% (95% CI, 43.5 to 65.1) for BEV + RT, P = .001. Treatment was well tolerated. There were a 5% rate of acute grade 3+ treatment-related AEs and no delayed high-grade AEs. Most patients died of recurrent GBM.
Conclusion: To our knowledge, NRG Oncology/RTOG1205 is the first prospective, randomized multi-institutional study to evaluate the safety and efficacy of re-RT in recurrent GBM using modern RT techniques. Overall, re-RT was shown to be safe and well tolerated. BEV + RT demonstrated a clinically meaningful improvement in PFS, specifically the 6-month PFS rate but no difference in OS.
Trial registration: ClinicalTrials.gov NCT01730950.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987-996, 2005 - PubMed
-
- Walker MD, Green SB, Byar DP, et al. : Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 303:1323-1329, 1980 - PubMed
-
- Laing RW, Warrington AP, Graham J, et al. : Efficacy and toxicity of fractionated stereotactic radiotherapy in the treatment of recurrent gliomas (phase I/II study). Radiother Oncol 27:22-29, 1993 - PubMed
-
- Navarria P, Navarria P, Minniti G, et al. : Re-irradiation for recurrent glioma: Outcome evaluation, toxicity and prognostic factors assessment. A multicenter study of the Radiation Oncology Italian Association (AIRO). J Neurooncol 142:59-67, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous