BAY-069, a Novel (Trifluoromethyl)pyrimidinedione-Based BCAT1/2 Inhibitor and Chemical Probe
- PMID: 36261130
- PMCID: PMC9661481
- DOI: 10.1021/acs.jmedchem.2c00441
BAY-069, a Novel (Trifluoromethyl)pyrimidinedione-Based BCAT1/2 Inhibitor and Chemical Probe
Abstract
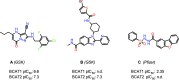
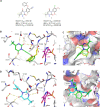
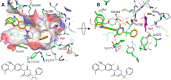
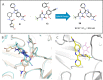
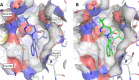
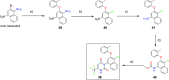
The branched-chain amino acid transaminases (BCATs) are enzymes that catalyze the first reaction of catabolism of the essential branched-chain amino acids to branched-chain keto acids to form glutamate. They are known to play a key role in different cancer types. Here, we report a new structural class of BCAT1/2 inhibitors, (trifluoromethyl)pyrimidinediones, identified by a high-throughput screening campaign and subsequent optimization guided by a series of X-ray crystal structures. Our potent dual BCAT1/2 inhibitor BAY-069 displays high cellular activity and very good selectivity. Along with a negative control (BAY-771), BAY-069 was donated as a chemical probe to the Structural Genomics Consortium.
Conflict of interest statement
The authors declare the following competing financial interest(s): J.G., R.C.H., K.Z., S.K., C.L., D.N., C.L., R.N., U.G., M.D., H.R., and L.B. are or have been employees and stockholders of Bayer AG.
Figures

References
-
- Tönjes M.; Barbus S.; Park Y. J.; Wang W.; Schlotter M.; Lindroth A. M.; Pleier S. V.; Bai A. H. C.; Karra D.; Piro R. M.; Felsberg J.; Addington A.; Lemke D.; Weibrecht I.; Hovestadt V.; Rolli C. G.; Campos B.; Turcan S.; Sturm D.; Witt H.; Chan T. A.; Herold-Mende C.; Kemkemer R.; König R.; Schmidt K.; Hull W. E.; Pfister S. M.; Jugold M.; Hutson S. M.; Plass C.; Okun J. G.; Reifenberger G.; Lichter P.; Radlwimmer B. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat. Med. 2013, 19, 901–908. 10.1038/nm.3217. - DOI - PMC - PubMed
-
- Thewes V.; Simon R.; Hlevnjak M.; Schlotter M.; Schroeter P.; Schmidt K.; Wu Y.; Anzeneder T.; Wang W.; Windisch P.; Kirchgäßner M.; Melling N.; Kneisel N.; Büttner R.; Deuschle U.; Sinn H. P.; Schneeweiss A.; Heck S.; Kaulfuss S.; Hess-Stumpp H.; Okun J. G.; Sauter G.; Lykkesfeldt A. E.; Zapatka M.; Radlwimmer B.; Lichter P.; Tönjes M. The branched-chain amino acid transaminase 1 sustains growth of antiestrogen-resistant and ERα-negative breast cancer. Oncogene 2017, 36, 4124–4134. 10.1038/onc.2017.32. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Molecular Biology Databases

