Mechanism of an intramembrane chaperone for multipass membrane proteins
- PMID: 36261528
- PMCID: PMC7614104
- DOI: 10.1038/s41586-022-05336-2
Mechanism of an intramembrane chaperone for multipass membrane proteins
Abstract
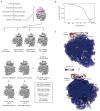
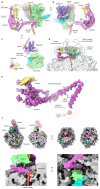
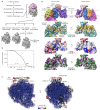
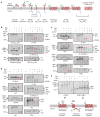
Multipass membrane proteins play numerous roles in biology and include receptors, transporters, ion channels and enzymes1,2. How multipass proteins are co-translationally inserted and folded at the endoplasmic reticulum is not well understood2. The prevailing model posits that each transmembrane domain (TMD) of a multipass protein successively passes into the lipid bilayer through a front-side lateral gate of the Sec61 protein translocation channel3-9. The PAT complex, an intramembrane chaperone comprising Asterix and CCDC47, engages early TMDs of multipass proteins to promote their biogenesis by an unknown mechanism10. Here, biochemical and structural analysis of intermediates during multipass protein biogenesis showed that the nascent chain is not engaged with Sec61, which is occluded and latched closed by CCDC47. Instead, Asterix binds to and redirects the substrate to a location behind Sec61, where the PAT complex contributes to a multipass translocon surrounding a semi-enclosed, lipid-filled cavity11. Detection of multiple TMDs in this cavity after their emergence from the ribosome suggests that multipass proteins insert and fold behind Sec61. Accordingly, biogenesis of several multipass proteins was unimpeded by inhibitors of the Sec61 lateral gate. These findings elucidate the mechanism of an intramembrane chaperone and suggest a new framework for multipass membrane protein biogenesis at the endoplasmic reticulum.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures









Comment in
-
Assembly surprise for membrane proteins.Nature. 2022 Nov;611(7934):40-41. doi: 10.1038/d41586-022-03221-6. Nature. 2022. PMID: 36261716 No abstract available.
References
-
- von Heijne G. The membrane protein universe: what’s out there and why bother? J Intern Med. 2007;261:543–57. - PubMed
-
- Heinrich SU, Mothes W, Brunner J, Rapoport TA. The Sec61p complex mediates the integration of a membrane protein by allowing lipid partitioning of the transmembrane domain. Cell. 2000;102:233–44. - PubMed
-
- Görlich D, Rapoport TA. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

