Metformin improves tendon degeneration by blocking translocation of HMGB1 and suppressing tendon inflammation and senescence in aging mice
- PMID: 36262012
- PMCID: PMC10113400
- DOI: 10.1002/jor.25470
Metformin improves tendon degeneration by blocking translocation of HMGB1 and suppressing tendon inflammation and senescence in aging mice
Abstract
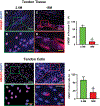
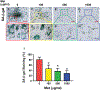
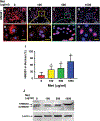
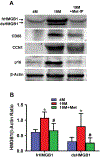
This study aimed to characterize aging-induced tendinopathy in mouse Achilles tendon and also to assess the treatment effects of metformin (Met) on aging tendon. We showed that compared to young tendon, aging tendon was in an inflammatory and senescent state as shown by increased expression of inflammatory disulfide HMGB1 (dsHMGB1), inflammatory macrophage marker CD68, and senescent cell markers SA-β-gal, p53, and p16. Moreover, aging tendon was degenerated marked by accumulation of proteoglycans and lipids in its interior. However, treatment of aging tendon by intraperitoneal (IP) injection of Met, a specific inhibitor of HMGB1, reduced dsHMGB1 levels, decreased the expression of CD68, SA-β-gal, CCN1, and p16 in vitro and in vivo. Furthermore, Met treatment also increased the number of NS, SSEA-1, and CD73 positive stem cells in culture and improved the tendon structure in aging mouse. These findings of this study indicate that Met exerts anti-inflammatory and anti-senescent effects on aging tendon.
Keywords: HMGB1; aging; degeneration; inflammation; metformin; senescence; tendon.
© 2022 Orthopaedic Research Society. Published by Wiley Periodicals LLC.
Figures








Similar articles
-
Effect of Metformin on Development of Tendinopathy Due to Mechanical Overloading in an Animal Model.Foot Ankle Int. 2020 Dec;41(12):1455-1465. doi: 10.1177/1071100720966318. Epub 2020 Nov 12. Foot Ankle Int. 2020. PMID: 33180557 Free PMC article.
-
Metformin decreases LPS-induced inflammatory response in rabbit annulus fibrosus stem/progenitor cells by blocking HMGB1 release.Aging (Albany NY). 2019 Nov 26;11(22):10252-10265. doi: 10.18632/aging.102453. Epub 2019 Nov 26. Aging (Albany NY). 2019. PMID: 31772144 Free PMC article.
-
Celecoxib, Beyond Anti-inflammation, Alleviates Tendon-Derived Stem Cell Senescence in Degenerative Rotator Cuff Tendinopathy.Am J Sports Med. 2022 Jul;50(9):2488-2496. doi: 10.1177/03635465221098133. Epub 2022 Jun 6. Am J Sports Med. 2022. PMID: 35666137
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
-
HMGB1: a potential new target for tendinopathy treatment.Connect Tissue Res. 2023 Jul;64(4):362-375. doi: 10.1080/03008207.2023.2199089. Epub 2023 Apr 9. Connect Tissue Res. 2023. PMID: 37032550 Review.
Cited by
-
Metformin lotion as a novel approach to prevent tendinopathy induced by mechanical overuse.Sci Rep. 2025 Aug 26;15(1):31474. doi: 10.1038/s41598-025-16279-9. Sci Rep. 2025. PMID: 40858887 Free PMC article.
-
Metformin increases the expression of proinflammatory cytokines and inhibits supraspinatus fatty infiltration.J Orthop Surg Res. 2023 Sep 12;18(1):674. doi: 10.1186/s13018-023-04163-z. J Orthop Surg Res. 2023. PMID: 37700364 Free PMC article.
-
Mitigating Scar Tissue Formation in Tendon Injuries: Targeting HMGB1, AMPK Activation, and Myofibroblast Migration All at Once.Pharmaceuticals (Basel). 2023 Dec 17;16(12):1739. doi: 10.3390/ph16121739. Pharmaceuticals (Basel). 2023. PMID: 38139865 Free PMC article.
-
Insulin-like growth factor-1 infusion in preterm piglets does not affect growth parameters of skeletal muscle or tendon tissue.Exp Physiol. 2024 Sep;109(9):1529-1544. doi: 10.1113/EP092010. Epub 2024 Jul 9. Exp Physiol. 2024. PMID: 38980930 Free PMC article.
-
Patellar tendon biomechanical and morphologic properties and their relationship to serum clinical variables in persons with prediabetes and type 2 diabetes.J Orthop Res. 2024 Aug;42(8):1653-1669. doi: 10.1002/jor.25816. Epub 2024 Feb 23. J Orthop Res. 2024. PMID: 38400550 Free PMC article.
References
-
- Colby SL, Ortman JM. 2015. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. In: Commerce editor; pp. 1–13.
-
- Zhou B, Zhou Y, Tang K. 2014. An overview of structure, mechanical properties, and treatment for age-related tendinopathy. J Nutr Health Aging 18:441–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

