Native valve, prosthetic valve, and cardiac device-related infective endocarditis: A review and update on current innovative diagnostic and therapeutic strategies
- PMID: 36263017
- PMCID: PMC9574252
- DOI: 10.3389/fcell.2022.995508
Native valve, prosthetic valve, and cardiac device-related infective endocarditis: A review and update on current innovative diagnostic and therapeutic strategies
Abstract
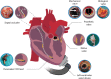
Infective endocarditis (IE) is a life-threatening microbial infection of native and prosthetic heart valves, endocardial surface, and/or indwelling cardiac device. Prevalence of IE is increasing and mortality has not significantly improved despite technological advances. This review provides an updated overview using recent literature on the clinical presentation, diagnosis, imaging, causative pathogens, treatment, and outcomes in native valve, prosthetic valve, and cardiac device-related IE. In addition, the experimental approaches used in IE research to improve the understanding of disease mechanisms and the current diagnostic pipelines are discussed, as well as potential innovative diagnostic and therapeutic strategies. This will ultimately help towards deriving better diagnostic tools and treatments to improve IE patient outcomes.
Keywords: biofilm; cardiac device; diagnosis; infection; infective endocarditis; native valve; prosthetic valve; treatment.
Copyright © 2022 Kouijzer, Noordermeer, van Leeuwen, Verkaik and Lattwein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abdelhady W., Bayer A. S., Seidl K., Nast C. C., Kiedrowski M. R., Horswill A. R., et al. (2013). Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus . Antimicrob. Agents Chemother. 57, 1447–1454. 10.1128/aac.02073-12 - DOI - PMC - PubMed
-
- Abikhzer G., Martineau P., Grégoire J., Finnerty V., Harel F., Pelletier-Galarneau M. (2020). [18F]FDG-PET CT for the evaluation of native valve endocarditis. J. Nucl. Cardiol. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

