Engineered red blood cells (activating antigen carriers) drive potent T cell responses and tumor regression in mice
- PMID: 36263022
- PMCID: PMC9573954
- DOI: 10.3389/fimmu.2022.1015585
Engineered red blood cells (activating antigen carriers) drive potent T cell responses and tumor regression in mice
Abstract
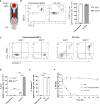
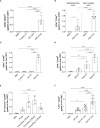
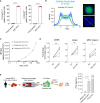
Activation of T cell responses is essential for effective tumor clearance; however, inducing targeted, potent antigen presentation to stimulate T cell responses remains challenging. We generated Activating Antigen Carriers (AACs) by engineering red blood cells (RBCs) to encapsulate relevant tumor antigens and the adjuvant polyinosinic-polycytidylic acid (poly I:C), for use as a tumor-specific cancer vaccine. The processing method and conditions used to create the AACs promote phosphatidylserine exposure on RBCs and thus harness the natural process of aged RBC clearance to enable targeting of the AACs to endogenous professional antigen presenting cells (APCs) without the use of chemicals or viral vectors. AAC uptake, antigen processing, and presentation by APCs drive antigen-specific activation of T cells, both in mouse in vivo and human in vitro systems, promoting polyfunctionality of CD8+ T cells and, in a tumor model, driving high levels of antigen-specific CD8+ T cell infiltration and tumor killing. The efficacy of AAC therapy was further enhanced by combination with the chemotherapeutic agent Cisplatin. In summary, these findings support AACs as a potential vector-free immunotherapy strategy to enable potent antigen presentation and T cell stimulation by endogenous APCs with broad therapeutic potential.
Keywords: CD8 T cells; activating antigen carriers; adjuvant; antigen presenting cell; cancer immunotherapy; dendritic cells; human papillomavirus; red blood cells.
Copyright © 2022 Blagovic, Smith, Ramakrishnan, Moore, Soto, Thompson, Stockmann, Kruszelnicki, Thakkar, Murray, Torres, Wondimagegnhu, Yi, Dadgar, Paracha, Page, Clear, Chaudhry, Myint, Bridgen, Gilbert, Seidl, Sharei, Loughhead, Bernstein and Yarar.
Conflict of interest statement
All listed authors are current or former SQZ Biotechnologies Company employees.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

